Abstract
Cladophialophora carrionii is one of the four major etiologic agents of human chromoblastomycosis in semi-arid climates. This species was studied using sequence data of the internal transcribed spacer region of rDNA, the partial β-tubulin gene and an intron in the translation elongation factor 1-alpha gene, in addition to morphology. With all genes a clear bipartition was observed, which corresponded with minute differences in conidiophore morphology. A new species, C. yegresii, was introduced, which appeared to be, in contrast to C. carrionii, associated with living cactus plants. All strains from humans, and a few isolates from dead cactus debris, belonged to C. carrionii, for which a lectotype was designated. Artificial inoculation of cactus plants grown from seeds in the greenhouse showed that both fungi are able to persist in cactus tissue. When reaching the spines they produce cells that morphologically resemble the muriform cells known as the “invasive form” in chromoblastomycosis. The tested clinical strain of C. carrionii proved to be more virulent in cactus than the environmental strain of C. yegresii that originated from the same species of cactus, Stenocereus griseus. The muriform cell expressed in cactus spines can be regarded as the extremotolerant survival phase, and is likely to play an essential role in the natural life cycle of these organisms.
Keywords: Cactus, chromoblastomycosis, Cladophialophora, endophyte, extremotolerance, phylogeny, taxonomy
INTRODUCTION
Cladophialophora carrionii (Trejos) de Hoog, Kwon-Chung & McGinnis is one of the most frequent etiologic agents of human chromoblastomycosis, a chronic cutaneous disease characterised by verrucose skin lesions eventually leading to emerging, cauliflower-like eruptions. The species is particularly observed in arid and semi-arid climates of e.g. South and Central America (Lavelle 1980) and Australia (Trejos 1954, Riddley 1957). The current hypothesis is that patients suffering from chromoblastomycosis are rural workers who acquire the infection after being pricked by cactus thorns or splinters (Rubin et al. 1991, Fernández-Zeppenfeldt et al. 1994). A classical case reported by O'Daly (1943) concerns traumatic inoculation with thorns of “guazábara” (Opuntia caribaea), a common xerophilic plant in semi-arid Venezuela. This hypothesised traumatic route of infection was later supported by Richard-Yegres & Yegres (Richard-Yegres & Yegres 1987; strain SR3 = CBS 863.96) and Fernández-Zeppenfeldt et al. (1994), who isolated strains from Prosopis juliflora litter. Cladophialophora Borelli has also been detected in association with spines of the common xerophyte Aloe vera and of the Cactaceae: Opuntia caribaeae, O. caracasana, Stenocereus griseus and Cereus lanuginosus (Borelli 1972, Yegres et al. 1996). Thorny American cacti are important components of the xerophyte flora of the arid climate of our study area in Falcon State, Venezuela (Richard-Yegres et al. 1992). Muriform cells are produced in human skin and represent the supposed pathogenic invasive form of fungi causing chromoblastomycosis (Mendoza et al. 1993). Early experiments involving the inoculation of several species of cold-blooded animals have shown the abundant production of the characteristic muriform cells in vivo (Trejos 1953).
A similar plant origin of chromoblastomycosis has been supposed for a related agent of chromoblastomycosis, Fonsecaea pedrosoi (Brumpt) Negroni. Marques et al. (2006) isolated this species from the shells of Babassu coconuts (Orbignya phalerata). The habit of local people to sit on these shells might explain the frequent occurrence of lesions on the buttocks (Silva et al. 1995). Salgado et al. (2004) found the species on the thorns of a Mimosa pudica plant which a patient could identify as the source of traumatic onset of his chromoblastomycosis.
Recently, with the development of molecular tools for species identification, doubt has arisen about the correctness of this supposed route of infection. The question whether environmental and clinical strains represent exactly the same species needs to be re-determined. In order to establish this for C. carrionii-associated chromoblastomycosis, reference strains from the CBS culture collection, supplemented with a large set of strains from semi-arid Venezuela, have been verified using molecular tools that are currently routinely employed to answer taxonomic questions in black yeasts and their filamentous relatives (de Hoog et al. 2003), particularly the internal transcribed spacer (ITS) region of rDNA, the partial β-tubulin gene (BT2), and an intron in the translation elongation factor 1-alpha (EF1). In addition, a series of three experiments has been conducted concerning inoculation into and superficial application onto germlings of Stenocereus griseus obtained by cultivation in vitro, mature plants of S. griseus from the wild, and in spines of S. griseus collected in the semi-arid area of study. Our aim is to reveal the role of the cactus S. griseus in the life cycle of its associated Cladophialophora spp., and to determine whether a link could be made to C. carrionii for obtaining a better understanding of human chromoblastomycosis.
MATERIALS AND METHODS
Fungal strains and morphology
Strains studied are listed in Table 1. This list comprises strains which have morphologically been identified as C. carrionii. Reference strains from the CBS culture collection, as well as fresh isolates from patients and the environment have been included. Strains were lyophilised and stored in liquid nitrogen soon after deposit at CBS. Stock cultures for transient working collections were grown on slants of 2 % malt extract agar (MEA) and oatmeal agar (OA) at 24 °C. For morphological observation, slide cultures were made of strains grown on potato-dextrose agar (PDA) (de Hoog et al. 2000) and mounted in lactophenol cotton blue.
Table 1.
Isolation data of Cladophialophora strains examined.
| Name | CBS nr. | Other reference(s)1 | GenBank | mtDNA* (Kawasaki et al. 1993) | Source [human: duration, localization, sex, age (Pérez-Blanco et al. 2003)]. | Geography | |
|---|---|---|---|---|---|---|---|
| ITS, BT2, EF1 | |||||||
| A/I/1: C. carrionii | |||||||
| 117904 | UNEFM 0004-02 = dH 14480 | EU137281, -,- | Chromoblastomycosis; 14 y; hip, thigh, leg; male 38 | Falcon State, Venezuela | |||
| 117891 | UNEFM 0002-00 = dH 14475 | EU137278, -, EU137222 | Chromoblastomycosis; 1 y; male 62 | Falcon State, Venezuela | |||
| 117906 | UNEFM 0014-96 = dH 14504 | EU137288, EU137171, EU137231 | Chromoblastomycosis; 0.5 y; hand; male 45 | Falcon State, Venezuela | |||
| 117897 | UNEFM 0011-03 = dH 14497 | EU137314, -, EU137254 | Chromoblastomycosis; 0.5 y; hand; male 42 | Falcon State, Venezuela | |||
| 859,96 | UNEFM 9617 = dH 10703 | EU137295, EU137178, EU137237 | Dry plant debris, arid zone | Falcon State, Venezuela | |||
| 117898 | UNEFM 0010-98 = dH 14496 | EU137308, -, EU137246 | Chromoblastomycosis; 20 y; hand; female 59 | Falcon State, Venezuela | |||
| 117889 | UNEFM 0003-04 = dH 14478 | -, EU137190, - | Chromoblastomycosis; 20 y; thigh, leg; female 78 | Falcon State, Venezuela | |||
| 114392 | UNEFM 82267 = dH 13261 | EU137267, EU137150, EU137211 | Chromoblastomycosis; leg; female | Falcon State, Venezuela | |||
| 114394 | UNEFM 9803 = dH 13263 | EU137307, -, EU137245 | Chromoblastomycosis; hand; male 22 | Falcon State, Venezuela | |||
| 114396 | UNEFM 2001/1 = dH 13265 | EU137269, EU137152, EU137213 | Chromoblastomycosis; arm; male 35 | Falcon State, Venezuela | |||
| 114399 | UNEFM 2003/2 = dH 13268 | EU137272, EU137155, EU137216 | Chromoblastomycosis; arm; female 64 | Falcon State, Venezuela | |||
| 114401 | UNEFM 9901 = dH 13270 | EU137274, EU137157, EU137218 | Chromoblastomycosis; arm; female 40 | Falcon State, Venezuela | |||
| 114402** | UNEFM 9902 = dH 13271 | EU137275, EU137158, EU137219 | Chromoblastomycosis; arm; female 40 | Falcon State, Venezuela | |||
| 114403 | UNEFM 95195 = dH 13272 | EU137276, EU137159, EU137220 | Chromoblastomycosis; arm; male | Falcon State, Venezuela | |||
| 117899 | UNEFM 0010-04 = dH 14495 | EU137301, EU137183, EU137241 | Chromoblastomycosis; 2 y; hand; male 57 | Falcon State, Venezuela | |||
| 117901 | UNEFM 0009-03 = dH 14492 | EU137312, EU137197, EU137252 | Chromoblastomycosis; 8 y; arm; female 41 | Falcon State, Venezuela | |||
| 114393 | UNEFM 9801 = dH 13262 | EU137268, EU137151, EU137212 | Chromoblastomycosis; hand; male 72 | Falcon State, Venezuela | |||
| 108.97** | UNEFM 9501 = dH 10704 | EU137306, EU137188, EU137265 | Chromoblastomycosis; skin | Falcon State, Venezuela | |||
| 114397 | UNEFM 84020 = dH 13266 | EU137270, EU137153, EU137214 | Chromoblastomycosis; hand, arm; male 54 | Falcon State, Venezuela | |||
| 114404 | UNEFM 95656 = dH 13273 | EU137311, EU137196, EU137251 | Chromoblastomycosis; arm; male | Falcon State, Venezuela | |||
| 117902 | UNEFM 0008-03 = dH 14489 | EU137283, EU137166, EU137226 | Chromoblastomycosis; 3 y; arm; male 42 | Falcon State, Venezuela | |||
| 117893 | UNEFM 0001-00 = dH 14470 | EU137316, EU137200, - | Chromoblastomycosis; 2 y; knee; male 19 | Falcon State, Venezuela | |||
| 117892 | UNEFM 0001-02 = dH 14471 | EU137277, EU137160, EU137221 | Chromoblastomycosis; 8 y; knee; male 52 | Falcon State, Venezuela | |||
| 117908 | UNEFM 0013-04 = dH 14502 | -, EU137191, - | Chromoblastomycosis; 6 y; back; male 13 | Falcon State, Venezuela | |||
| 109.97** | UNEFM 9503 = dH 10706 | -, -, - | Chromoblastomycosis; skin | Falcon State, Venezuela | |||
| 857.96 | UNEFM 9408 = dH 10707 | EU137294, EU137177, EU137236 | Chromoblastomycosis; skin | Falcon State, Venezuela | |||
| 114398 | UNEFM 2003/1 = dH 13267 | EU137271, EU137154, EU137215 | Chromoblastomycosis; arm; female 67 | Falcon State, Venezuela | |||
| 114400 | UNEFM 2003/3 = dH 13269 | EU137273, EU137156, EU137217 | Chromoblastomycosis; arm; male 50 | Falcon State, Venezuela | |||
| 117909 | UNEFM 0013-00 = dH 14501 | EU137287, EU137170, EU137230 | Chromoblastomycosis; arm; male | Falcon State, Venezuela | |||
| 114395 | UNEFM 9802 = dH 13264 | EU137299, EU137182, EU137240 | Chromoblastomycosis; leg; female 22 | Falcon State, Venezuela | |||
| 166.54 | MUCL 10088 | EU137290, EU137173, - | Skin lesion in human | Falcon State, Venezuela | |||
| 862.96 | UNEFM 9603 = dH 10700 | EU137315, EU137199, EU137255 | Dry plant debris, semi-arid zone | Falcon State, Venezuela | |||
| 863.96** | IFM 41444 = UNEFM SR3 = dH 10699 | AB109169 / EU137296, EU137179, EU137238 | Dry spine (Opuntia caribaea) on soil, semi-arid zone | Falcon State, Venezuela | |||
| 861.96 | UNEFM 9607 = dH 10701 | EU137309, EU137194, EU137249 | Dry plant debris, semi-arid zone | Falcon State, Venezuela | |||
| 117896 | dH 14498 | EU137285, -, EU137228 | Hand lesion | Falcon State, Venezuela | |||
| 114397 | UNEFM 84020 = dH 13266 | EU137270, EU137153, EU137214 | Chromoblastomycosis, hand and arm | Falcon State, Venezuela | |||
| 117905 | dH 14505 | EU137300, -, - | Chromoblastomycosis, hand, male | Falcon State, Venezuela | |||
| 117900 | dH 14493 | EU137284, -, EU137227 | Chromoblastomycosis, hand, male | Falcon State, Venezuela | |||
| 114392 | UNEFM 82267 = dH 13261 | EU137267, EU137150, EU137211 | Chromoblastomycosis, leg, female | Falcon State, Venezuela | |||
| - | FMC 248 | AF397181, -, - | Chromoblastomycosis | Venezuela | |||
| - | IFM 41807 | AB109175, -, - | Group mt-I | - | Venezuela | ||
| - | IFM 4812 | AB109168, -, - | Group mt-I | - | Venezuela | ||
| - | IMTSP 690 | AF397180, -, - | Chromoblastomycosis | Brazil | |||
| 410.96 | UAMH 4392 = NCMH 1010 = DUKE 2403 | EU137310, EU137195, EU137250 | Chromoblastomycosis | - | |||
| 163.54 | EU137304, EU137186, EU137243 | Chromoblastomycosis | Australia | ||||
| 117903 | dH 14482 | EU137282, -, EU137225 | Chromoblastomycosis, forearm, male | - | |||
| 362.70 | M.J. Campos 4555 = dH 15806 | EU137302, EU137184, EU137242 | Human | Mozambique | |||
| 260.83 | CDC B-1352 = FMC 282 = ATCC 44535 (ex-T of C. ajelloi) | EU137292, EU137175, EU137234 | Group mt-I | Skin lesion in human | Uganda | ||
| 986.96 | UAMH 5717 | EU137297, EU137180, - | Clinical material | - | |||
| - | IFM 4805 | AB087204, -, - | - | - | |||
| - | IFM 4811 | AB109178, -, - | - | - | |||
| - | IFM 41814 | AB109176, -, - | - | - | |||
| B/II/2: C. carrionii | |||||||
| 160.54 | ATCC 16264 = CDC A-835 = MUCL 40053 = IFM 4808 (ex-LT of C. carrionii) | AB109177 / EU137266, EU137201, EU137210 | Group mt-II | Chromoblastomycosis, human | Australia | ||
| - | Todd Pryce 200867 = dH 13218 | Human | Australia | ||||
| 406.96 | MRL 1114 = UAMH 4366 = dH 15847 | EU137317, EU137202, EU137256 | Human | Queensland, Australia | |||
| 100434 | ATCC 32279 = dH 10745 = IP 518 = RV 16499 | EU137289, EU137172, EU137232 | Human | Madagascar | |||
| - | IFM 4810 | AB109170, -, - | - | - | |||
| - | IFM 41446 = DCU 606 | AB109171, -, - | - | - | |||
| C: C. carrionii | |||||||
| - | IFM 41651 | AB109174, -, - | Group mt-I | - | China | ||
| - | IFM 41650 | AB109173, -, - | Group mt-I | - | China | ||
| - | IFM 41641 | AB109172, -, - | Group mt-I | - | China | ||
| - | IFM 4985 | AB109179, -, - | - | - | |||
| - | IFM 4986 | AB109180, -, - | - | - | |||
| D / III / 3: C. yegresii | |||||||
| 114406 | UNEFM SgSR1 = dH 13275 | EU137323, EU137208, EU137263 | Stenocereus griseus asymptomatic plant | Falcon State, Venezuela | |||
| 114407 | UNEFM SgSR2 = dH 13276 | EU137324, -, EU137264 | Stenocereus griseus asymptomatic plant | Falcon State, Venezuela | |||
| 114405** | UNEFM SgSR3 = dH 13274 (ex-T of C. yegresii) | EU137322, EU137209, EU137262 | Stenocereus griseus asymptomatic plant | Falcon State, Venezuela | |||
Abbreviations: ATCC = American Type Culture Collection, Manassas, U.S.A.; CBS = Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands; CDC = Centers for Disease Control and Prevention, Atlanta, U.S.A.; DCU = Department of Dermatology, School of Medicine, Chiba, Japan; dH = G.S. de Hoog working collection; FMC = Faculdade de Medicina, Caracas, Venezuela; ITMSP = Instituto de Medicina Tropical de São Paulo, São Paulo, Brazil; IFM = Research Center for Pathogenic Fungi and Microbial Toxicoses, Chiba University, Chiba, Japan; IP = Institut Pasteur, Paris, France; MUCL = Mycotheque de l'Université de Louvain, Louvain-la-Neuve, Belgium; RV = Prince Leopold Institute of Tropical Medicine, Antwerp, Belgium; UAMH = The University of Alberta Microfungus Collection and Herbarium, Edmonton, Canada; UNEFM = Universidade Nacional Experimental Francisco de Miranda, Coro, Falcon, Venezuela.
Ex-T = Type strain; ex-LT = Lectotype strain.
Type I and II groups based on mitochondrial DNA restriction fragment length polymorphism: H-1 and H-2 = restriction patterns 1 and 2, respectively, with HaeIII enzyme; M-1 and M-2 = restriction patterns 1 and 2, respectively, with MspI enzyme; S-1 and S-2 = restriction patterns 1 and 2, respectively, with Sau3AI enzyme.
Used in plant and mouse inoculation experiments.
DNA extraction
Approximately 1 cm2 mycelium of 30-d-old cultures was transferred to a 2 mL Eppendorf tube containing 300 μL TES-buffer (Tris 1.2 % w/v, Na-EDTA 0.38% w/v, SDS 2 % w/v, pH 8.0) and about 80 mg of a silica mixture (Silica gel H, Merck 7736, Darmstadt, Germany/Kieselguhr Celite 545, Machery, Düren, Germany, 2 : 1, w/w). Cells were disrupted mechanically in a tight-fitting sterile pestle for approximately 1 min. Subsequently 200 μL TES-buffer was added, the mixture was vortexed, 10 μL proteinase K was added and incubated for 10 min at 65 °C. After addition of 140 μL of 5 M NaCl and 1/10 vol CTAB 10 % (cetyltrimethylammoniumbromide) buffer, the material was incubated for 30 min at 65 °C. Subsequently 700 μL SEVAG (24 : 1, chloroform : isoamylalcohol) was mixed to solution, incubated during 30 min on ice water and centrifuged for 10 min at 14 000 rpm. The supernatant was transferred to a new tube with 225 μL 5 M NH4-acetate, incubated on ice water and centrifuged again for 10 min at 14 000 rpm. The supernatant was transferred to another Eppendorf tube with 0.55 vol isopropanol and spun for 5 min at 14 000 rpm. Subsequently, the pellet was washed with ice cold 70 % ethanol. After drying at room temperature it was re-suspended in 48.5 μL TE buffer (Tris 0.12 % w/v, Na-EDTA 0.04 % w/v) plus 1.5 μL RNAse 20 U/mL and incubated for 15-30 min at 37 °C.
Sequencing and phylogenetic reconstruction
Three loci, namely the internal transcribed spacers (ITS), ß-tubulin (BT2) and translation elongation factor 1-α (EF1), were sequenced. For ITS sequencing, amplification was performed with V9G (5'-TTACGTCCCTGCCCTTTGTA-3') and LS266 (5'-GCATTCCCAAACAACTCGACTC-3'). Sequencing reactions were conducted with ITS1 and ITS4 primers (White et al. 1990). For BT2 amplification and sequencing, primers Bt2a (5'-GGTAACCAAATCGGTGCTGCTTTC-3') and Bt2b (5'-ACCCTCAGTGTAGTGACCCTTGGC-3') were used (Glass & Donaldson 1995) and for EF1 amplification and sequencing, primers EF1-728F (5'CATCGAGAAGTTCGAGAAGG-3') and EF1-986R (5'-TACTTGAAGGAACCCTTACC-3') (Carbone & Kohn, 1999). Sequences were aligned in BioNumerics v. 4.5 (Applied Maths, Kortrijk, Belgium), exported and converted into Phylip interleaved format (Felsenstein 1993).
Calculation of ILD (incongruence length difference) was performed in PAUP v. 4.0b10 (Swofford 2003). A combined data set of ITS, EF1 and BT2 sequences was created. Optimality criterion was set to parsimony. The total number of characters was 1 263 with equal weight, while 677 characters were constant, and 396 parsimony-informative. Gaps were treated as missing, and tree-bisection-reconnection (TBR) was used as branch-swapping algorithm. Maximum number of trees was set to 100 and left unchanged.
Substitution model testing
The program MrAic (www.abc.se/~nylander/; Nylander 2004) was used to select a substitution model. MrAic is a Perl script for calculating the Akaike Information Criterion (AIC), corrected Akaike Information Criterion (AICc), Bayesian Information Criterion (BIC), and Akaike weights for nucleotide substitution models and model uncertainty. Using an ML algorithm, likelihood scores under different models were estimated using Phyml (http://atgc.lirmm.fr/phyml/). All 56 models implemented in Modeltest (Posada & Crandall 1998) were evaluated. These models were also combined with proportion of invariable sites (I) and/or gamma distribution shape parameter (G). A difference between Modeltest and MrAic is that the latter does not evaluate all models on the same, approximate topology as in PAUP (Swofford 1981). Instead, Phyml was used to try to find the maximum of the likelihood function under all models. This is necessary for finding AIC, AICc, or BIC for the models. The AICc calculation (Table 2) was used to select the right model for the ratio of parameters to characters (Nchar/Nparameters < 40; Burnham & Anderson 2002) for all loci. The substitution matrix of the models is printed next to the trees. Another advantage of using MrAic in combination with Phyml was the obtained accuracy of tree topology and the greater calculation speed (Guindon & Gascuel 2003).
Table 2.
Results from MrAic using corrected Akaike Information Criterion (AICc).
| Fragment/Gene | Model | df* | InL* | AICc* | wAICc* |
|---|---|---|---|---|---|
| rRNA ITS | TrNG | 89 | −21.840.556 | 4575.9992″ | 0.3080 |
| EF-1α | HKYG | 88 | −19.392.355 | 41.147.171 | 0.5242 |
| β-Tubulin | SYMIG | 90 | −28.547.745 | 59.261.933 | 0.1913 |
df = degrees of freedom; InL = log likelihood; AICc = corrected AIC; wAICc = weighted corrected AIC.
Population genetic analyses
In order to confirm the intraspecific diversity shown in the MP trees, the number of populations in the C. carrionii complex was inferred with Structure v. 2.2 (Pritchard et al. 2000) using genotype data of the ITS regions of rRNA gene and of the partial EF1 and BT2 genes. Genotypes of these three loci of 43 isolates were sorted on the basis of sequence similarity. Structure is a model-based clustering method for using multilocus genotype data to infer population structure and assign individuals to populations. The parameters were as follows: the length of burn-in period was set to 106, number of MCMC repeats after burn-in 30 000; the ancestry model: admixture (individuals have mixed ancestry and is recommended as starting point for most analyses). Uniform prior for ALPHA was set to 1.0 (default) and all allele frequencies were taken as independent among populations with λ set to 1.0 (default). Probability of the data (for estimating K) was also computed (Falush et al. 2003). The burn-in period length and number of MCMC repetitions after burn-in were set as 10 000 and 100 000, and admixture model and allele frequencies correlated model were chosen for analysis. The number of populations (K) was assumed from two to four.
Association of multilocus genotypes was screened with the multilocus option in BioNumerics. To test for reproductive mode in each population, index of association (IA, a measure of multilocus linkage disequilibrium) was calculated with Multilocus v. 1.2.2 (www.bio.ic.ac.uk/evolve/software/multilocus). The null hypothesis for this analysis was complete panmixia. The values of IA were compared between observed and randomised data sets. The hypothesis would be rejected when p < 0.05. Population differentiation (index: theta, θ) was also detected using the same software and a null hypothesis for this analysis is no population differentiation. When observed θ is statistically significantly different from those of random datasets (p < 0.05), population differentiation should be considered.
A reticulogram was reconstructed using T-rex (Makarenkov 2001, Makarenkov & Legendre 2004) (www.labunix.uqam.ca/~makarenv/trex.html) on C. carrionii/Cladophialophora sp. The program first computed a classical additive tree using one of the five available tree reconstruction algorithms. Subsequently, at each step of the procedure, a reticulation (a new edge) was chosen that minimised the least-squares or the weighted least-squares loss function; it was added to the growing reticulogram. Two statistical criteria (Q1 and Q2) were proposed to measure the gain in fit when reticulations were added. The minimum of each of these criteria may suggest a stopping rule for addition of reticulations. With HGT (horizontal gene transfer) reticulogram reconstruction option (Makarenkov 2001) the program mapped the gene tree into the species tree using the least-squares method. Horizontal transfers of the considered gene were then shown in the species tree. The reticulate network was created in the ITS tree, which served as a species tree and compared with a gene tree, EF1. Degrees of recombination or horizontal gene transfer were also visualised using SplitsTree v. 4.8 software (Huson & Bryant 2006). Split decomposition (Bandelt & Dress 1992) was applied on three loci of the entire C. carrionii complex. Calculation was done with default settings of characters transformation using uncorrected P-values, equal angles and optimise box iterations set to 1. Star- or brush-like trees indicate clonal development, while reticulation indicates genetic exchange.
Isolation of fungi for inoculation experiments
Nine plants of Stenocereus griseus, located within 50 m radius of the house of a patient with chromoblastomycosis due to strain UNEFM 9902 = CBS 114402 (C. carrionii) in Sabaneta (Miranda, Falcón State, Venezuela), were analysed. Four fragments of approx. 2 × 3 × 1 cm were excised from each plant at brownish superficial lesions in upper branches. Sampled fragments were soaked in mineral oil for 15 min at 23 °C under agitation at 150 rpm (Fernández-Zeppenfeldt et al. 1994). Subsequently four cultivations were made per sample on agar slants. Strains with cultural characteristics and morphology similar to C. carrionii (de Hoog et al. 2000) were selected. Final identification was made by sequencing of the ITS region, by determining the ability of strains to grow at 35, 37, 38 and 40 °C, and whether they could break down 20 % gelatin (Richard-Yegres & Yegres 1987, Fernández-Zeppenfeldt et al. 1994). Environmental strain UNEFM-SgSR3 = CBS 114405 (Cladophialophora sp.) and clinical strain UNEFM 9902 = CBS 114402 (C. carrionii) were selected for the inoculation experiments.
Inoculum preparation
Approximately 1 cm2 of a culture on Sabouraud's glucose agar (SGA) was transferred to 50 mL YPG medium (yeast extract 0.5 %, peptone 0.5 %, glucose 2 %) (de Hoog et al. 2000), shaken at 150 rpm and incubated for 3 d at 23 °C (Yegres et al. 1991). Five mL aliquots of the starter culture were transferred serially every 4 d to 500 mL flasks containing 100 mL synthetic medium (d-glucose 2 %, KH2PO4 0.2 %, NH4SO4 0.1 %, urea 0.03 %, MgSO4 0.03 %, CaCl2 0.003 %; pH 6.2) shaken at 150 rpm at 23 °C. After 4 d the suspensions, which were predominantly conidia, were filtered through sterile gauze, ground in 50 mL 0.85 % saline, centrifuged at 2 000 rpm, and repeatedly washed with saline until a clear supernatant was obtained. The suspensions were adjusted to 5 × 106 cells/mL (Yegres et al. 1991, Cermeño & Torres 1998). Inocula of 2 mL were checked for viability in lactritmel medium (de Hoog et al. 2000).
Experimental cactus germlings
Young cactus plants (Stenocereus griseus) were obtained in the laboratory (Clausnitzer 1978) by cultivation from seeds of a single cardon fruit collected near the house of the patient infected with CBS 114402 in the endemic area for chromoblastomycosis in Falcon State, Venezuela. The seeds were rinsed with sterile distilled water, the contents washed by agitation for 10 min at 120 rpm in 250 mL sodium hypochlorite 4 % (v/v), and subsequently with sterile distilled water at 120 rpm for 5 min. The supernatant was decanted, 250 mL HCl 20 % was added, the seeds were incubated for 3 h, decanted and washed repeatedly with sterile distilled water. Seeds were then dried for 24 h on filter paper at 37 °C. Onset of germination was obtained by incubation of the seeds in a moist chamber on filter paper for 15 d under alternately 8 h of continuous white light (26 W) and 16 h of darkness; bud emergence was observed daily. Germlings of 1 cm in length, with green colour and having two leaves were transplanted to 128-container germinators until roots developed. The sterile substrate contained 5 parts Sogemix® and 1 part river soil from the region where the fruit was collected. The daily light regime was as above; plants were watered every 10 d with 5 mL sterile tap water for 1 yr.
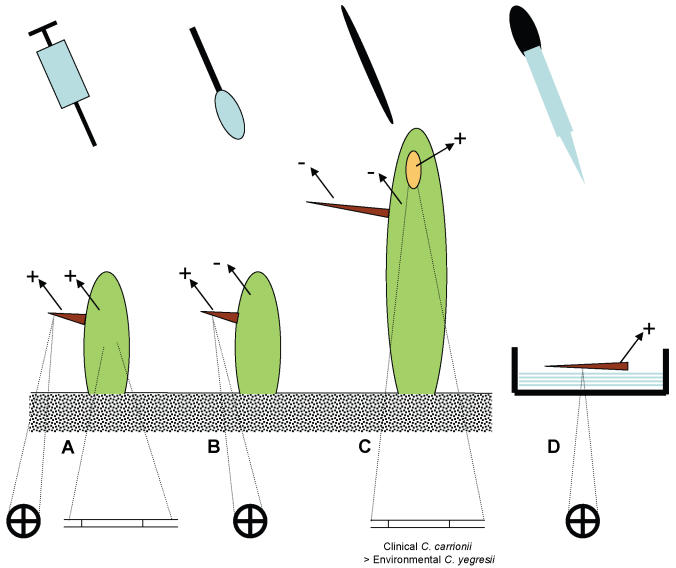
Inoculation of S. griseus germlings
Fungal suspensions (0.1 mL) were either injected using a syringe (13 × 0.4 mm) at a depth of approximately 5 mm into cortical tissue (Fig. 1A), or superficially applied onto (Fig. 1B) 96 randomly selected 1-yr-old plants: 50 % using clinical strain CBS 114402 (C. carrionii) and 50 % using environmental strain CBS 114405 (Cladophialophora sp.). The controls were 64 plants which were treated similarly, but using sterile saline (0.85 %). The growth chambers with inoculated plants stayed in the laboratory under the conditions specified above. From day 15 post inoculation onwards every 15th day, six plants of each treatment were sectioned longitudinally from the apex and transversely by means of a hand-held microtome, examined directly in glycerin water (25 %), and cultured in lactritmel medium (Fernández-Zeppenfeldt et al. 1994).
Fig. 1.
Diagram of inoculation experiments with results. A. Inoculation of young cactus; B. Superficial application of young cactus; C. Traumatic application of mature cactus, with brown resulting scar; D. Superficial application of mature spines. Indications +/- refer to positive resp. negative results of re-isolated strains. Lower line: circles represent production of muriform cells, filaments represent hyphal growth.
Experimental cactus plants
A total of 150 whole S. griseus plants ≤ 15 cm tall and without macroscopically visible lesions, were dug from an area within a 50 m radius of the house of the patient with chromoblastomycosis as specified above. Plants were transported to the laboratory and transplanted individually into polyethylene bags with a capacity of 1 kg, using as substrate river soil from the same area. Plants were maintained outside, directly adjacent to the laboratory to adjust at average temperatures of 32 °C and with natural daylight. They were watered with tap water every 15th d for a period of 6 mo.
Scar formation in mature S. griseus plants
For inoculation purposes, 150 sharp, wooden toothpicks 4 × 0.3 × 0.2 cm were washed and boiled for 3 min in tap water to eliminate resins (Yegres & Richard-Yegres 2002). This procedure was repeated three times. Three batches of 50 toothpicks each were kept separate in Petri dishes. Plates were incubated for 15 d at 23 °C after inoculating each batch with 1 mL fungal suspension (5 × 106 cells/mL) of either strain CBS 114405 or CBS 114402, with sterile water as control. A total of 50 randomly selected plants were inoculated (Fig. 1C) halfway up the shaft with a toothpick colonised with CBS 114402 (C. carrionii), CBS 114405 (Cladophialophora sp.) or the control (Yegres & Richard-Yegres 2002).
Starting from 2 wk post inoculation, 10 plants were randomly chosen every 15 d, and tissue samples taken at the point of inoculation, and from the thorns directly adjacent to this area. Samples were rinsed with 4 % sodium hypochlorite for 3 min, and subsequently washed in sterile distilled water for re-isolations, and for histological study by means of light microscopy (Fernández-Zeppenfeldt et al. 1994).
Experiments with spines of S. griseus
Ninety cactus spines of 2.5 cm av. length were collected from a single S. griseus plant located near the home of the patient infected with CBS 114402, at approx. 2.5 m height, superficially sterilised, and divided into three groups, of which 30 spines were inoculated with CBS 114402 (C. carrionii), 30 with CBS 114405 (Cladophialophora sp.) and 30 to be used as control, inoculated with a saline solution (Fig. 1D). A similar series composed of 90 spines of 1.5 cm average length was collected at approx. 1 m height. All spines were incubated in sterile Petri dishes with filter paper (Whatman #1) with 2 mL saline solution; subsequently 0.1 mL fungal suspension was applied. Twenty spines were analysed weekly by means of longitudinal sectioning with a hand-held microtome, cultured as above and observed microscopically until day 75 post incubation.
Statistics
Survival of the cactus seedlings and collected plants following inoculation were evaluated using the X2-test (P = 0.05 was considered significant). Stem lesions resulting from inoculations were analysed with Student's T-test (P = 0.01 was considered significant).
RESULTS
The rDNA ITS region was sequenced for 43 strains identified as C. carrionii based on morphology. Sequences of 16 additional strains were downloaded from GenBank. Five distantly related, unidentified cladophialophora-like species were added, with CBS 834.96 as outgroup. In C. carrionii, 203 positions were compared in ITS1, 158 in the 5.8S rRNA gene and 182 in ITS2 (Table 3). The sequences could be aligned with confidence over their entire lengths. Over the data set, 35 positions were polymorphic, of which 33 were phylogenetically informative (Table 3), the two remaining being variable T-repeats near the ends of ITS1 and 2.
Table 3.
Nucleotide variability of ITS1-2 ribosomal DNA regions of Cladophialophora carrionii (A - C) and C. yegresii (D).
rDNA domains (length), with variable nucleotide positions.
| ITS1 (201-203) | A | B | C | D | |
|---|---|---|---|---|---|
| 16 | C | C | C | T | |
| 17 | T | C | T | T | |
| 19 | T | T | T | C | |
| 51 | A | A | A | G | |
| 57 | A | A | A | T | |
| 90-92 | TG- | TG- | TG- | CGT | |
| 101 | T | T | T | C | |
| 103 | C | C | C | T | |
| 104 | G | A | G | G | |
| 106 | A | A | A | G | |
| 114 | T | T | T | C | |
| 122 | T | T | T | C | |
| 132 | C | C | C | T | |
| 137 | A | A | A | C | |
| 141 | C | C | C | T | |
| 145 | - | - | - | A | |
| 163-170 | 6-10T | 6-10T | 6-10T | TTGTATCT | |
| 180 | - | - | - | A | |
| 183 | G | G | G | A | |
| 190 | T/A | A | A | A | |
| 5.8S (158) | Monomorphic | ||||
| ITS2 (178-182) | A | B | C | D | |
| 36 | C | T | T | T | |
| 48 | T | T | G | T | |
| 49 | T | T | T | C | |
| 51 | - | - | - | C | |
| 114 | C | C | C | G | |
| 140 | A | A | A | G | |
| 155 | - | - | - | T | |
| 178-179 | -- | -- | -- | CT | |
For ITS sequences the AICc selected the TrN+G model (TrNG; Tamura & Nei 1993). The base frequency of ITS: T = 0.2467, C = 0.2897, A = 0.2247, G = 0.2390, TC = 0.5364, AG = 0.4636. The EF1 tree was built with substitution model HKY+G; the base frequency of EF1: T = 0.2990, C = 0.2665, A = 0.2123, G = 0.2221, TC = 0.5655, AG = 0.4345. The best model for BT2 sequences was the SYM+I+G (symmetrical model). The base frequency for BT2: T = 0.2255, C = 0.2953, A = 0.2463, G = 0.2328, TC = 0.5208, AG = 0.4792. Bootstrap values of the EF1 tree were calculated with PAUP using parsimony and with maxtrees set to 500 and 500 replicates (data not shown). Total number of characters was 191 of which 101 were parsimony-informative. Tree length was 365 and had the following indices: Consistency Index = 0.685, Retention Index = 0.542 and Homoplasy Index = 0.315.
The original tree length, Lo was 1 055, the tree length of the combined data, Lc was 1 062. The resulting incongruence length difference L = (Lc-Lo) was 7 (P = 0.24). The observed ILD was not significantly greater than expected by chance and it was concluded that the sequences were congruent and could be used together in a combined analyses.
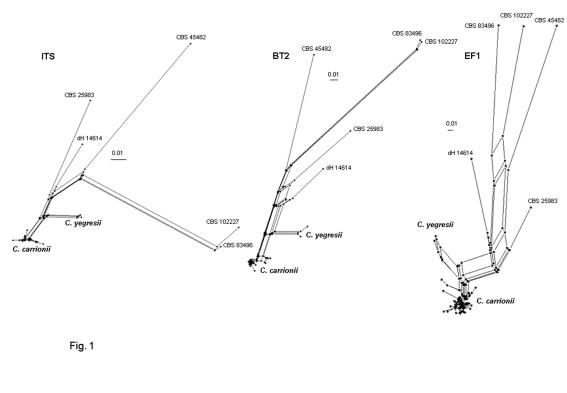
Split decomposition based on the same alignment generated extensive recombination. The structure found with three loci was robust, with the exception of separation of CBS 834.96 and CBS 102227 with EF1 (Fig. 2).
Fig. 2.
Split decomposition of the C. carrionii complex using SplitsTree with uncorrected (P-value) distances. Nodes are shown only with different genotypes; hence EF1 shows the largest number of nodes. Extensive reticulation is noted in all loci.
The core of the network, comprising the strains listed in Table 1, was analysed in more detail. With ITS, four groups were recognised (A-D; Table 1). (A) was the main group with 36 strains/sequences; FMC 248 differed only by a small T-repeat and was regarded as a member of (A). The remaining groups were smaller, differing from group (A) maximally by two consistent positions (Table 3). Group (C) mainly comprised sequences from GenBank and all originated from Abliz et al. (2004). One of the strains of group (C), IFM 4808, concerned a subculture of CBS 160.54, which is an original isolate of Trejos (1954) representing C. carrionii. Re-sequencing indicated that it was a member of group (B). Analysis of our electropherograms of this isolate was not suggestive of heterothallism. None of the positions characterising groups (A)-(C) were also found to differ in group (D), which deviated in 16 mutations in ITS1 and 8 in ITS2; 17 of the mutations were transitions, 7 were transversions and 7 indels. Group (D) was clearly distinct from the complex of (A)-(C), with a total of 27 mutations.
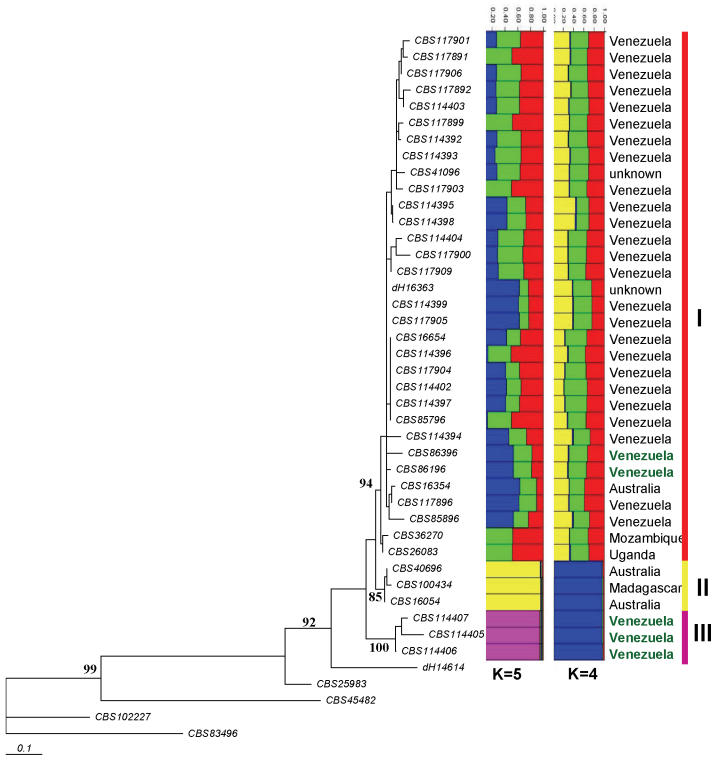
For multilocus analysis with ITS, EF1 and BT2 a smaller set of strains was compared. Sequences of the 205 bp long element of EF1 contained 32 phylogenetically informative mutations. Three entities were distinguished (I-III; Fig. 3). With BT2, three groups with the same composition were recognised. Strains of ITS group (C) were not available for study.
Fig. 3.
Phylogenetic tree (Neighbour-joining) of the C. carrionii complex based on EF1 (with grouping I-III) using the same strains of Fig. 1, generated using the HKY+G model. The model was calculated using ML in MrAic software. Bootstrap cut-off = 80 %. CBS 834.96 was taken as outgroup. Columns were generated with Structure software hypothesising K = 4 and K = 5, and alleles independent. Geographical origins in black refer to isolates from humans (chromoblastomycosis); origins in green refer to isolates from plant material.
On the basis of multilocus screening in BioNumerics, concordant groups (A)-(D) were tested with the Structure programme. When K was set at 4 or 5, consistent groupings were noted, indicated as I, II and III (Fig. 3), corresponding with ITS groups (A), (B) and (D), respectively in Table 1.
The possibility that group (D)/(III) included a member of another, morphologically similar but phylogenetically unrelated group of fungi was excluded by SSU sequencing. Genera morphologically similar to Cladophialophora, such as Cladosporium Link, Devriesia Seifert & N.L. Nick., Phaeoramularia Munt.-Cvetk., Pseudocladosporium U. Braun and Stenella Syd. proved to be remote (data not shown).
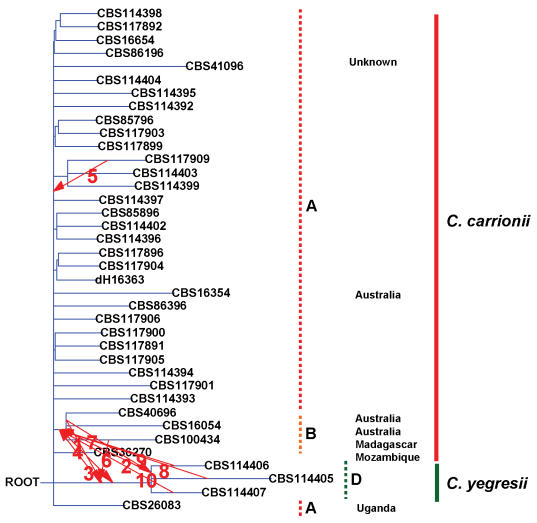
With T-rex, interaction between groups (B) and (D) was noted, rather than between groups (B) and (A), despite the high sequence similarity of (A) and (B) (Fig. 4).
Fig. 4.
Reticulogram of South American strains of Cladophialophora species and strains from other continents (mentioned) constructed with T-rex software. ITS rDNA (with grouping A, B, D) was used as species tree and compared with the ß-tubulin gene tree. First 10 reticulations are shown with numbers.
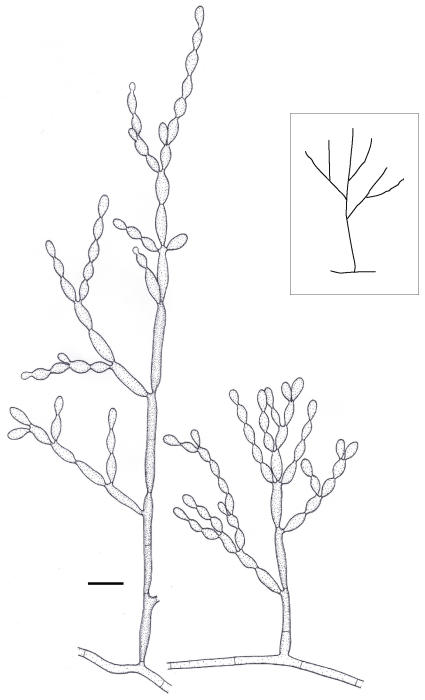
Morphological observation revealed that representatives of ITS groups (A)-(C) generally had conidiophores that arise at right angles from creeping hyphae (Fig. 5), while those of (D) tend to be ascending, hyphae gradually becoming conidiophore-like. Since slight correspondence was found in independent markers and phenetic criteria, we considered group (D) to represent a separate species, which is described as follows.
Fig. 5.
Microscopic morphology of C. carrionii, strain CBS 160.54. Conidiophore erect, i.e. mostly arising at 90° from creeping hypha (sketch). Scale bar = 10 μm.
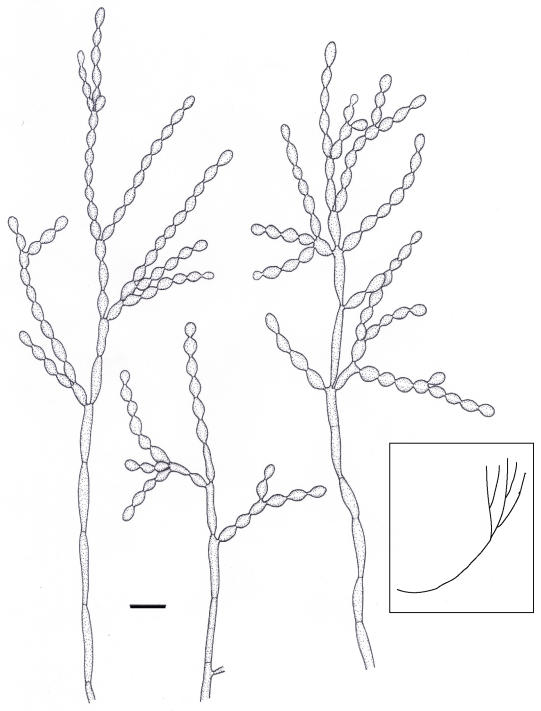
Cladophialophora yegresii de Hoog, sp. nov. MycoBank MB500208. Figs 6, 7D-F.
Fig. 6.
Microscopic morphology of C. yegresii, strain CBS 114405. Conidiophore ascending, i.e. mostly emerging from hyphal end that is gradually growing upwards to become a conidiophore (sketch). Scale bar = 10 μm.
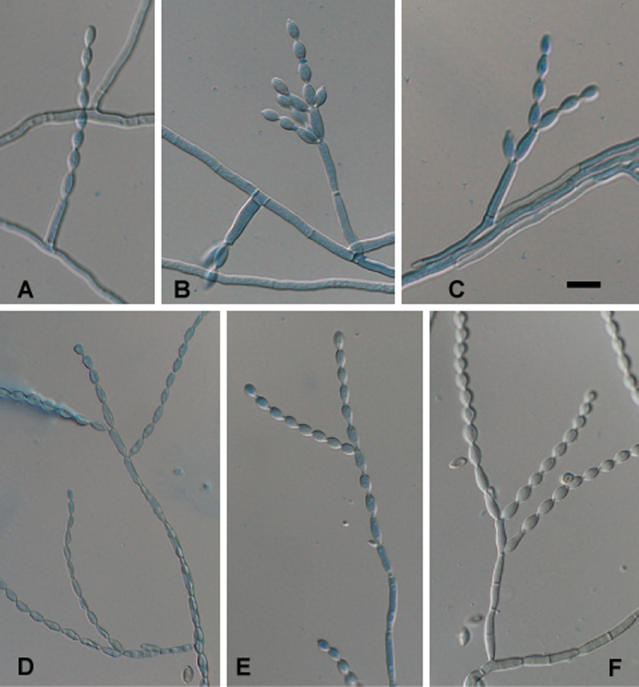
Fig. 7.
Conidial morphology in selected branches of (upper row: A-C) C. carrionii, strain CBS 260.83; (lower row: D-F) C. yegresii, strain CBS 114405. In this respect the two species are identical. Scale bar = 10 μm.
Etymology: Named after Francisco Yegres, Venezuelean mycologist.
Coloniae in agaro PDA dicto 22 °C planae, olivaceo-virides, pulverulentae vel velutinae, margine integra; reversum olivaceo-atrum. Hyphae fertiles dilute olivaceo-virides, ascendentes, paulatim in catenas conidiorum concolorium vertentes. Conidiorum catenae ramosae, conidia dilute olivaceo-viridia, levia et tenuitunicata, 4.5-6 × 2.5 μm, faciliter liberata, cicatricibus modice pigmentatis. Chlamydosporae et cellulae zymosae absentes. Synanamorphe phialidica non visa. Teleomorphe ignota.
Holotypus cultura sicca CBS H-18464 in herbarium CBS praeservatur.
Colonies on PDA at 22 °C evenly olivaceous green, powdery to velvety, with entire margin; reverse olivaceous black. Fertile hyphae pale olivaceous green, ascending, gradually changing over into concolorous chains of conidia. Conidial system profusely branched. Conidia pale olivaceous green, smooth- and thin-walled, 4.5-6 × 2.5 μm, detached rather easily, with slightly pigmented scars. Chlamydospores and yeast cells absent. Phialidic synanamorph not observed. Teleomorph unknown.
Specimen examined: Venezuela, Falcon state, from asymptomatic Stenocereus griseus cactus, G. Fernández-Zeppenfeldt, CBS H-18464 holotype, culture ex-type CBS 114405 = UNEFM SgSR3.
Notes: Of the 48 dematiaceous isolates obtained from 36 fragments of the cactus Stenocereus griseus, four strains originating from four different plants of S. griseus presented morphological and physiological key characteristics of Cladophialophora carrionii or C. yegresii (de Hoog et al. 2000, 2006). Gelatin liquefaction was negative in all strains and the maximum growth temperature was 37 °C. After identification to species level using sequence data (de Hoog et al. 2006), both C. carrionii and C. yegresii appeared to be among the strains isolated.
A total of 256 plants obtained at the end of 1 yr from germlings, had ribs, spines, and an average height of 15 cm. The 96 germlings inoculated with fungal suspensions of the test strains CBS 114402 (C. carrionii, clinical) and CBS 114405 (C. yegresii, environmental) remained without visible external lesions during the year of experimentation. Histological sections of the 96 inoculated plants consistently revealed internal growth of the fungi in their filamentous form. Muriform cells were not observed, neither on the epidermis, nor in the internal tissue, spines or roots. The re-isolated cultures demonstrated the viability of the fungi during the entire experimental process: CBS 114402 (C. carrionii) was grown from 26 (54.16 %) of the plants and CBS 114405 (C. yegresii) in 23 (47.90 %) of the plants. The X2 test did not reveal significant differences between the isolates (X2c = 0.0729 < X2t = 3.84). The 32 control plants remained without external lesions, and in the histological sections no internal or external fungal elements were observed. None of the fungi isolated from the control plants proved to be a species of Cladophialophora.
With 96 plants with superficial application of spore suspension (48 plants for each isolate, either clinical or environmental) neither internal nor external lesions were observed. Histological sectioning did not reveal fungal elements in or on plant tissue. Short hyphal elements and meristematic cells were occasionally seen around and inside the outer layers of the spines. The re-isolated strains proved that the fungi survived during the entire experimental procedure: CBS 114402 (C. carrionii) was isolated from 32 (66.67 %) plants and CBS 114405 (C. yegresii) from 33 (68.75 %). The X2 test did not detect significant differences in survival rates among the isolates (X2c = 0.4375 < X2t = 3.84).
Mature plants inoculated using colonised toothpicks showed average scarring of 1.88 cm diam with C. carrionii and 1.33 cm diam with C. yegresii, around the point of inoculation. In histological sections of 100 plants, dark, septate hyphae with inflated elements were observed at the points of inoculation. Muriform cells were not observed. Re-isolated strains were evidence of isolate viability: CBS 114402 (C. carrionii) was grown from 36 (72 %) plants and CBS 114405 (C. yegresii) from 30 (60 %). The fungi could not be isolated from spines. The 50 plants used as controls showed scarring of 1.06 cm diam on average around the point of inoculation. No fungal elements were seen in direct examinations and histological sections of these plants. The retro-cultures were negative. The scarring responses of the plants to the clinical strain, environmental strain and control proved to be highly significant:
Clinical CBS 114402 vs. environmental CBS 114405: þ = 0.000832, P = 0.01;
Clinical CBS 114402 vs. control: þ = 0.00003128, P = 0.01;
Environmental CBS 114405 vs. control: þ = 0.005343, P = 0.01.
Spines 2.5 cm av. in length, seeded with suspensions of CBS 114402 (C. carrionii) and CBS 114405 (C. yegresii), developed toruloid hyphal elements with some dark, swollen cells similar to muriform cells known in human tissue. The re-isolated strains proved the species to survive during the experimental procedure (< 75 d). Similar results were obtained with the spines 1.5 cm av. in length. No cladophialophora-like fungi were isolated from the controls.
DISCUSSION
Taxonomy of Cladophialophora
Judging from SSU rDNA phylogeny data, all Cladophialophora species that are consistently associated with pathology to humans belong to the Herpotrichiellaceae in the order Chaetothyriales (Haase et al. 1999). Within this order, the genus Cladophialophora is polyphyletic. Conidia of all species are produced in branched chains on poorly differentiated hyphae. This very simply structured conidial system may lead to confusion with morphologically similar but unrelated fungi that are encountered as contaminants in the hospital environment. The genus Cladosporium comprises ubiquitous airborne fungi which mostly have erect, more or less differentiated conidiophores, and dark conidial scars. They are associated with Davidiella Crous & U. Braun teleomorphs and belong to the Dothideomycetes, family Davidiellaceae (Braun et al. 2003, Schoch et al. 2006). Pseudocladosporium was introduced by Braun (1998) with three species differing from Cladophialophora mainly by intercalary hyphal cells with lateral extensions that bear conidial chains, having Caproventuria U. Braun teleomorphs (see Crous et al. 2007 - this volume). The group is classified in the Venturiaceae and Mycosphaerellaceae in the Dothideales (Braun et al. 2003). The anamorph genus Devriesia comprises thermophilic saprobes with a cladophialophora-like appearance and producing dark, multi-celled chlamydospores alongside the hyphae. Phylogenetically this genus is related to the Mycosphaerellaceae, in the Dothideomycetes (Seifert et al. 2004).
Cladophialophora carrionii was originally introduced by Trejos (1954) on the basis of 46 strains from Venezuela, Australia and South Africa. He did not indicate a holotype. For this reason isolate Trejos 27 = Emmons 8619 = CBS 160.54, the first strain mentioned by Trejos (1954), is selected here as representative for C. carrionii. A dried specimen of this strain has been deposited as lectotype in the Herbarium of the Centraalbureau voor Schimmelcultures as CBS H-18465.
The ex-type strain of Cladophialophora ajelloi Borelli, CBS 260.83, proved to be indistinguishable from C. carrionii, which was also known to be able to produce phialides in addition to catenate conidia (Honbo et al. 1984). Remarkably, a strain identified as C. ajelloi from Samoa (CBS 259.83; Goh et al. 1982) proved to be related to but consistently different from all strains of the C. carrionii complex. The 43-year-old male patient in otherwise good health carrying this fungus had a 5 × 3 cm erythematous, scaling lesion on his arm. Muriform cells were present in superficial dermis and stratum corneum. This clearly represents yet another agent of human chromoblastomycosis. The name C. ajelloi is not available for this taxon, as this is a synonym of C. carrionii. The taxon will be formally described in a forthcoming paper.
Members of ITS groups (A)-(D) were shown to be close to each other in SSU phylogeny (data not shown) underlining that all analysed species were correctly assigned to Cladophialophora. This genus was defined by melanised acropetal chains of conidia, near absence of conidiophores, and phylogenetic affinity to the order Chaetothyriales. Strains (A)-(D) clustered in a clade which contained a mixture of species of Cladophialophora, Fonsecaea Negroni and Phialophora Medlar. From a point of view of human disease, the species of the clade were known as agents of brain infection [C. bantiana (Sacc.) de Hoog et al., F. monophora (M. Moore & F.P. Almeida) de Hoog et al.], disseminated disease [C. devriesii (A.A. Padhye & Ajello) de Hoog et al.], cutaneous disease [C. boppii (Borelli) de Hoog et al.] and particularly chromoblastomycosis (C. carrionii, Fonsecaea, Phialophora).
Diversity of Cladophialophora carrionii/C. yegresii
Infraspecific variability was observed within C. carrionii. The groups (A)-(C) were separated on the basis of five mutations in the ITS region, which were supported by mutations in EF1 and BT2, as confirmed by analysis in Structure, where the same separation (K = 5) of entities was observed. Furthermore, K = 4 unites groups (B/II) and (D/III), despite the fact that the sequence of (B) is more close to those of (A). With T-rex software a similar relationship between [(B), C. carrionii] and [(D), C. yegresii] was noted, suggesting horizontal gene flow between these entities. This is remarkable, since (C) strains predominantly inhabit remote deserts in Madagascar and Australia, while (D) is found in equally remote localities in Venezuela. Extensive reticulation was observed in all genes with SplitsTree. With ITS and BT2, CBS 834.96 and CBS 102227 cluster closely together, while in the more variable EF1 data these are all widely apart, suggesting that in Cladophialophora other mechanisms than recombination may occur.
Group (C) contained ITS sequences taken from the public domain, originating from a single study (Abliz et al. 2004). Remarkably, strain IFM 4808 found in group (C) on the basis of data from Abliz et al. (2004), was the same isolate as CBS 160.54, which was found repeatedly in group (B) in our data set (Table 1). A similar phenomenon was observed with strain IFM 41444 = CBS 863.96, of which GenBank deposition AB109169 consistently deviated from our data in a frequently observed mutation. A possible explanation of these consistent sequence conflicts is heterozygosity. Although most chaetothyrialean fungi are supposed to be haploid (Szaniszlo 2002; Zeng et al. 2007), some strains have a double DNA content in yeast cells (Ohkusu et al. 1999). Teleomorphs are not known in Cladophialophora and related black yeasts, but many species are known to form profuse hyphal anastomoses (de Hoog et al. 2006), allowing parasexual processes and mitotic recombination. However, all electropherograms including those from the study of Abliz et al. (2004), which were kindly sent by K. Fukushima (Chiba, Japan), were unambiguous, without double peaks. This matched with the observation of preponderant clonality despite frequent anastomoses in Exophiala J.W. Carmich. (Zeng et al. 2007). An alternative explanation might be the occurrence of paralogous ITS repeats, as reported earlier in Fusarium Link (O'Donnell & Cigelnik 1997).
The remaining diversity within C. carrionii as confirmed by Structure shows some geographical structuring of populations, in that group (A) does not occur in Asia, group (B) is limited to Australia and Africa, and group (C) has thus far only been reported from Asia. The wide distribution of most genotypes suggests, however, that worldwide occurrence is likely to become apparent when more strains have been analysed. All climate zones where C. carrionii was isolated were semi-arid to arid, desert-like. Genotypes were not limited to the endemic semi-arid areas, and thus a relatively rapid vector of dispersal has to be hypothesised enabling the fungus to cross climate zones where the saprobic phase is unable to survive. Kawasaki et al. (1993) analysed three further loci in mtDNA using RFLP. Only some of their strains were available for sequencing. These had all identical mtDNA profiles, with the exception of IFM 4808 = CBS 160.54, that differed in two markers (Table 1). If we assume that there is no real separation of ITS groups (A) and (C) (see above), the conclusion is warranted that mtDNA allows distinction of polymorphism at the same level of diversity as detected in this study with ITS, EF1 and BT2.
South America harbours group (D) which represents a second species, C. yegresii. This species thus far has not been found on humans, and seems to be restricted to living Stenocereus cactus plants. Nishimura et al. (1989) published a strain from chromoblastomycosis in China which matched the morphology of strains now classified as C. yegresii, but as far as we are aware this strain has not been sequenced.
Ecology and virulence of Cladophialophora carrionii/C. yegresii
Cladophialophora carrionii was preponderantly found as an agent of human infection and only occasionally on dead plant debris, mainly seceded cactus needles. The only three strains available of C. yegresii were isolated from living, asymptomatic Stenocereus plants surrounding the cabin of a symptomatic patient from whom C. carrionii, CBS 114402 was isolated. Although in some publications convincing evidence was presented that infections originate from puncture by plant material (e.g., Salgado et al. 2004), it now becomes clear that the environmental look-alikes of clinical strains do not necessarily belong to the same species (Crous et al. 2006, Mostert et al. 2006), but may be members of other, related taxa with slightly different ecology; an unambiguous connection between a clinical and an environmental strain still has to be proven.
The endemic area of the two species, C. carrionii and C. yegresii, has a semi-arid climate, with average yearly temperatures of 24 °C, scarce rainfall (up to 600 annual mL) and is located at moderate altitude (up to 500 m) (Borelli 1979, Richard-Yegres & Yegres 1987). The landscape is dominated by large cacti and other xerophytes. Stenocereus griseus is a columnar American cactus with a very strong, protective external epidermis that allows the accumulation of water in the shaft and enables tolerance of extreme drought. The species produces ovoidal, thorny fruits of about 5 cm diam, which are commonly eaten by the local population. It has therefore been suggested that patients with chromoblastomycosis acquire their infection by traumatic inoculation with cactus spines, similar to the supposed infection process of Madurella mycetomatis (Laveran) Brumpt in the arid climate of Africa (Ahmed et al. 2002). The frequent occurrence of 16/1 000 for chromoblastomycosis in areas endemic for Cladophialophora in Venezuela (Yegres et al. 1985; Yegüez-Rodriguez et al. 1992) indicates a marked invasive potential for C. carrionii. Local goat-keepers are particularly at risk: in 1984, 14 of 18 patients investigated had these occupational characteristics (Yegres et al. 1985). Nevertheless, virulence of C. carrionii is low when inoculated into the footpads of mice (Yegres et al. 1998); also an environmental strain of C. carrionii failed to produce lesions in mice and in a volunteer (Richard-Yegres & Yegres 1987).
We performed inoculation experiments with C. carrionii and C. yegresii using freshly grown, healthy cacti in the greenhouse. The plants were followed over a 1-yr period; during all this time the control plants remained without lesions. Both Cladophialophora strains were able to produce infection when syringe-inoculated deep into young cactus tissue. Histopathology showed septate hyphae between host cells, and the shaft was maintained over prolonged periods without causing visible damage. This absence of appreciable destruction would categorise them as endophytes. Cactus tissue is rich in carbohydrates, vitamins and minerals (Vélez & Chávez 1980) which may promote endophyte growth.
In contrast, suspensions applied superficially lead to growth on and in spines only. The absence of infection after superficial application indicates that the fungi are unable to invade healthy plant tissue from the surface and thus they cannot be characterised as obligatory phytopathogens.
The two species differed in the degree of scarring after traumatic inoculation into mature plants: the clinical strain C. carrionii was consistently more virulent than C. yegresii that originated from the same host plant. Both species showed the same viability in re-isolated cultures. In nature, the fungi are likely to invade only when the integrity of the epidermis is broken, as happens e.g. by goat feeding or transmission by sap-sucking birds or piercing insects. They also show the same transformation to meristematic morphology (González et al. 1990) when entering hard spine tissue. A possible trigger for this conversion is the dominance of lignin in the spines. Survival on and in spines is enhanced by their capturing of atmospheric water formed after nightly condensation. The fact that superficial application leads to colonisation around and inside the spines suggests that the spines play a role in mechanic dispersion of the fungi.
A possibly coincidental mechanism of dispersal might be traumatic inoculation into living tissue of humans or animals, where the same muriform cells are formed, defining the skin disease chromoblastomycosis. It may be questioned whether animal/human inoculation plays a role in the evolution of the fungus. ITS differences between the two species are observed in 23 positions, with a ratio of transitions : transversions of 2 : 1 (Table 3). Thus no saturation of mutations has taken place and the diversification can be regarded as an example of recent sympatric speciation. Cladophialophora carrionii is widely distributed, and shows a higher degree of diversity than C. yegresii. This would be suggestive for a longer evolutionary time span of existence and C. carrionii then should be regarded as ancestral to C. yegresii, with the latter showing a founder effect due to the absence of polymorphisms. However, such an order of event (a host jump from humans to cactus) is difficult to imagine. It is more likely that C. yegresii is the original cactus endophyte exhibiting extremotolerance via its muriform cells. T-rex data suggested a more direct connection of C. yegresii with African and Australian rather than Venezuelean strains of C. carrionii. We suppose that the low degree of observed variation in C. yegresii is not a founder effect, but rather a sampling effect, as living cacti have thus far not been studied outside the framework of our study on the patient with Cladophialophora chromoblastomycosis. The difference in virulence may be simply explained by C. carrionii, which lives as a saprobe on dead cactus debris for part of its life cycle, and is less adapted to an endophytic life style.
Cladophialophora cf. carrionii is known to occur on lignified materials, such as wood chips of Eucalyptus crebra and wooden remains of Prosopis juliflora and Stenocereus griseus (Riddley 1957, Yegres et al. 1985, Fernández-Zeppenfeldt et al. 1994). This does not exclude a certain degree of pathogenicity to humans, as also pathogens like Cryptococcus neoformans (Sanfelice) Vuill. are known to have an essential part of their life cycle in hollows of Eucalyptus trees. Cryptococcus neoformans produces diphenol oxidase to degrade lignin, an aromatic polymer in the cell wall of plants and a component of wood (Cabral 1999). Similar degradation pathways are present in Cladophialophora carrionii (Prenafeta-Boldú et al. 2006).
The natural occurrence of C. carrionii and C. yegresii in association with xerophytes has been proven, but their environmental route of dispersal is still unknown. As transformation to meristematic cells takes place when the hyphae reach the spines and on dead spines, the muriform cell apparently is the extremotolerant phase of the species. The conidial anamorph can be found sporulating on rotten spines directly after rainfall (Richard-Yegres & Yegres 1987), but as the fungus has thus far never been isolated from outside air, it is still unclear how a new host plant is reached.
The behaviour of C. carrionii on humans, provoking the very characteristic disease, chromoblastomycosis, of which the agents are limited to the ascomycete family Herpotrichiellaceae (de Hoog et al. 2000) is puzzling. In humans, the extremophilic muriform anamorph is expressed rather than hyphae, and thus humans do not seem a natural reservoir of the fungus. Nevertheless some acquired cellular immunity seems to be involved. Albornoz et al. (1982) demonstrated that a significant share of the local population of goat keepers (Yegres et al. 1985) is asymptomatically infected with C. carrionii; Iwatsu et al. (1982) detected cutaneous delayed hypersensitivity in rats experimentally-infected with agents of chromoblastomycosis. With murine experimental infection of the related fungus Fonsecaea pedrosoi, Ahrens et al. (1989) found enlargement and metastasis of lesions in athymic but not in normal mice, or in mice with defective macrophage function. Several authors (Kurita 1979, Nishimura & Miyaji 1981, Polak 1984) observed a significant role of acquired cellular immunity in F. pedrosoi, while Cardona-Castro & Agudelo-Flórez (1999) obtained chronic infection in immunocompetent mice when inoculated intraperitoneally. Garcia Pires et al. (2002) noted an unbalance between protective Th1 and less efficient Th2 responses. The possible host response leads to different clinical types, referred to as tuberculoid and suppurative granuloma, respectively. The existence of genetic constitutional factors in susceptibility is underlined by a marked frequency of family relationships among symptomatic individuals (Yegüez-Rodriguez et al. 1992). The disease is not observed in local animals such as goats, possibly due to their high body temperature (≈ 39 °C). Nevertheless, hyphal fragments artificially inoculated into goats led to transformation into muriform cells, but the lesions disappeared within 60 d (Martínez et al. 2005). Further animal experiments using strains identified according to new taxonomy will be necessary to answer questions on the role of the fungus on warm-blooded animals.
Acknowledgments
The authors are indebted to F. Yegres, N. Richard-Yegres and V. Maigualida Pérez-Blanco for providing a large set of strains for study and information on strain ecology, and to R.C. Summerbell for helpful suggestions on the manuscript. P. Abliz and K. Fukushima are acknowledged for making sequence data available for study. K.F. Luijsterburg is thanked for technical assistance. The study was supported in part by the Fondo Nacional de Investigaciones Cientificas y Tecnológicas (Fonacit), Venezuela.
Taxonomic novelty: Cladophialophora yegresii de Hoog, sp. nov.
References
- Abliz P, Fukushima K, Takizawa K, Nishimura K (2004). Specific oligonucleotide primers for identification of Cladophialophora carrionii, a causative agent of chromoblastomycosis. Journal of Clinical Microbiology 42: 404-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed AOA, Adelmann D, Fahal A, Verbrugh H, Belkum A van, Hoog GS de (2002). Environmental occurrence of Madurella mycetomatis, the major agent of human eumycetoma in Sudan. Journal of Clinical Microbiology 40: 1031-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrens J, Graybill JR, Abishawl A, Tio FO, Rinaldi MG (1989). Experimental murine chromomycosis mimicking chronic progressive human disease. American Journal of Tropical Medicine and Hygiene 40: 651-658. [DOI] [PubMed] [Google Scholar]
- Albornoz MB, Marin C de, Iwatsu T (1982). Estudio epidemiologico de un area endemica para cromomicosis en el Estado Falcon. Investigación Clínica 23: 219-228. [Google Scholar]
- Bandelt HJ, Dress AW (1992). Split decomposition: a new and useful approach to phylogenetic analysis of distance data. Molecular Phylogenetics and Evolution 1: 242-252. [DOI] [PubMed] [Google Scholar]
- Borelli D (1972). Significado del dimorfismo de ciertos hongos parásitos. Mycopathologia et Mycologia Applicata 46: 237-239. [DOI] [PubMed] [Google Scholar]
- Borelli D (1979). Reservarea de algunos agentes de micosis. Medicina Cutánea 4: 367-370. [Google Scholar]
- Braun U (1998). A monograph of Cercosporella, Ramularia and allied genera (phytopathogenic hyphomycetes), Vol. 2. IHW-Verlag, Eching.
- Braun U, Crous PW, Dugan F, Groenewald JZ, Hoog GS de (2003). Phylogeny and taxonomy of Cladosporium-like hyphomycetes, including Davidiella gen. nov., the teleomorph of Cladosporium s. str. Mycological Progress 2: 3-18. [Google Scholar]
- Burnham KP, Anderson DR (2002). Model selection and multimodel inference, a practical information-theoretic approach. 2nd ed, Springer, New York.
- Cabral L (1999). Wood, animals and human beings as reservoirs for human Cryptococcus neoformans infection. Revista Iberoamericana de Micologia 16: 77-81. [PubMed] [Google Scholar]
- Carbone I, Kohn LM (1999). A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553-556. [Google Scholar]
- Cardona-Castro N, Agudelo-Flórez P (1999). Development of a chronic chromoblastomycosis model in immunocompetent mice. Medical Mycology 37: 81-83. [PubMed] [Google Scholar]
- Cermeño J, Torres J (1998). Método espectrofotométrico en la preparación del inóculo de hongos dematiaceos. Revista Iberoamericana de Micologia 15: 155-157. [PubMed] [Google Scholar]
- Clausnitzer I (1978). Germinación de las semillas del dato o frutos del cardón, Lemaireocereus griseus (Haw) Britt & Rose. Revista de la Facultad de Agronomia de la Universidad del Zulia 5: 351-365. [Google Scholar]
- Crous PW, Schubert K, Braun U, Hoog GS de, Hocking AD, Shin H-D, Groenewald JZ (2007). Opportunistic, human-pathogenic species in the Herpotrichiellaceae are phenotypically similar to saprobic or phytopathogenic species in the Venturiaceae. Studies in Mycology 58: 185-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Slippers B, Wingfield MJ, Rheeder J, Marasas WFO, Philips AJL, Alves A, Burgess T, Barber P, Groenewald JZ (2006). Phylogenetic lineages in the Botryosphaeriaceae. Studies in Mycology 55: 235-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK (2003). Inference of population structure: Extensions to linked loci and correlated allele frequencies. Genetics 164: 1567-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J (1993). PHYLIP (phylogeny inference package), version 3.6a2. Department of Genetics, Univ. Washington, Seattle.
- Fernández-Zeppenfeldt G, Richard-Yegres N, Yegres F, Hernández R (1994). Cladosporium carrionii: hongo dimórfico en cactáceas de la zona endémica para la cromomicosis en Venezuela. Revista Iberoamericana de Micologia 11: 61-63. [Google Scholar]
- Garcia Pires d'Ávila SC, Pagliari C, Seixas Duarte MI (2002). The cell-mediated immune reaction in the cutaneous lesion of chromoblastomycosis and their correlation with different clinical forms of the disease. Mycopathologia 156: 51-60. [DOI] [PubMed] [Google Scholar]
- Glass NL, Donaldson G (1995). Development of primer sets designed for use with PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh KS, Padhye AA, Ajello L (1982). A Samoan case of chromoblastomycosis caused by Cladophialophora ajelloi. Sabouraudia 20: 1-5. [PubMed] [Google Scholar]
- González R, Caleiras E, Guanipa O, Garcia-Tamayo J, Siva L, Colina C, Fernández-Zeppenfeldt G, Pacheco I (1990). Chromomycosis: ultrastructural characteristics of the suppurative granuloma in Cladosporium carrionii skin lesions. Jornada Microscopica Electrónica 4: 133-134. [Google Scholar]
- Guindon S, Gascuel O (2003). A simple, fast, and accurate algorithm to estimate phyogenies by maximum likelihood. Systematic Biology 52: 696-704. [DOI] [PubMed] [Google Scholar]
- Haase G, Sonntag L, Melzer-Krick B, Hoog GS de (1999). Phylogenetic interference by SSU-gene analysis of members of the Herpotrichiellaceae with special reference to human pathogenic species. Studies in Mycology 43: 80-97. [Google Scholar]
- Honbo S, Padhye AA, Ajello L (1984). The relationship of Cladosporium carrionii to Cladophialophora ajelloi. Sabouraudia 22: 209-218. [PubMed] [Google Scholar]
- Hoog GS de, Guarro J, Gené J, Figueras MJ (2000). Atlas of Clinical Fungi, 2nd ed. Centraalbureau voor Schimmelcultures, Utrecht and Universitat Rovira i Virgili, Reus.
- Hoog GS de, Vicente VA, Caligiorne RB, Kantarcioglu AS, Tintelnot K, Gerrits van den Ende AHG, Haase G (2003). Species diversity and polymorphism in the Exophiala spinifera clade containing opportunistic black yeast-like fungi. Journal of Clinical Microbiology 41: 4767-4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoog GS de, Zeng JS, Harrak MJ, Sutton DA (2006). Exophiala xenobiotica sp. nov., an opportunistic black yeast inhabiting environments rich in hydrocarbons. Antonie van Leeuwenhoek 90: 257-268. [DOI] [PubMed] [Google Scholar]
- Huson DH, Bryant D (2006). Application of phylogenetic networks in evolutionary studies. Molecular Biology and Evolution 23: 254-267. [DOI] [PubMed] [Google Scholar]
- Iwatsu T, Miyaji M, Taguchi H, Okamoto S (1982). Evaluation of skin test for chromoblastomycosis using antigens prepared from culture filtrates of Fonsecaea pedrosoi, Phialophora verrucosa, Wangiella dermatitidis and Exophiala jeanselmei. Mycopathologia 77: 59-64. [DOI] [PubMed] [Google Scholar]
- Kawasaki M, Ishizaki H, Miyaji M, Nishimura K, Matsumoto T, Honbo S, Muir D (1993). Molecular epidemiology of Cladosporium carrionii. Mycopathologia 124: 149-152. [DOI] [PubMed] [Google Scholar]
- Kurita N (1979). Cell-mediated immune responses in mice infected with Fonsecaea pedrosoi. Mycopathologia 68: 9-15. [DOI] [PubMed] [Google Scholar]
- Lavelle P (1980). Chromoblastomycosis in Mexico. Pan American Health Organization Scientific Publications 396: 235-247. [Google Scholar]
- Makarenkov V (2001). T-Rex: reconstructing and visualizing phylogenetic trees and reticulation networks. Bioinformatics 17: 664-668. [DOI] [PubMed] [Google Scholar]
- Makarenkov V, Legendre P (2004). From a phylogenetic tree to a reticulated network. Journal of Computational Biology 11: 195-212. [DOI] [PubMed] [Google Scholar]
- Marques SG, Pedroso Silva CM, Saldanha PC, Rezende MA, Vicente VA, Queiros-Telles F, Lopes Costa JM (2006). Isolation of Fonsecaea pedrosoi from the shell of the babassu coconut (Orbignya phalerata Martius) in the Amazon region of Maranhão Brazil. Japanese Journal of Medical Mycology 47: 305-311. [DOI] [PubMed] [Google Scholar]
- Martínez E, Rey Valieron C, Yergres F, Reyes R (2005). El caprino: aproximación a un modelo animal en la cromomicosis humana. Investigación Clínica 46: 131-138. [PubMed] [Google Scholar]
- Mendoza L, Karuppayil SM, Szaniszlo PJ (1993). Calcium regulates in vitro dimorphism in chromoblastomycotic fungi. Mycoses 36: 157-164. [DOI] [PubMed] [Google Scholar]
- Mostert L, Groenewald JZ, Summerbell RC, Gams W, Crous PW (2006). Taxonomy and pathology of Togninia (Diaporthales) and its Phaeoacremonium anamorphs. Studies in Mycology 54: 1-115. [Google Scholar]
- Nishimura K, Miyaji M (1981). Defense mechanisms of mice against Fonsecaea pedrosoi infection. Mycopathologia 76: 155-166. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Miyaji M, Taguchi H, Wang DL, Li RY, Meng ZH (1989). An ecological study on pathogenic dematiaceous fungi from China. In: Current problems of opportunistic fungal infections. Proceedings of the 4th International Symposium of the Research Center for Pathogenic Fungi and Microbial Toxicoses, Chiba University, Chiba: 17-20.
- Nylander JAA (2004). MrAic.pl. Programme distributed by the author. Evolutionary Biology Centre, Uppsala University.
- O'Daly JA (1943). La cromoblastomicosis en Venezuela. Memorias Primera Jornada Venezolana de Venereología. Caracas.
- O'Donnell K, Cigelnik E (1997). Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular and Phylogenetic Evolution 7: 103-116. [DOI] [PubMed] [Google Scholar]
- Ohkusu M, Yamaguchi M, Hata K, Yoshida S, Tanaka S, Nishimura K, Hoog GS de, Takeo K (1999). Cellular and nuclear characteristics of Exophiala dermatitidis. Studies in Mycology 43: 143-150. [Google Scholar]
- Polak A (1984). Experimental infection of mice by Fonsecaea pedrosoi and Wangiella dermatitidis. Sabouraudia 22: 167-169. [PubMed] [Google Scholar]
- Posada D, Crandall KA (1998). Modeltest: testing the model of DNA substitution. Bioinformatics 14: 817-818. [DOI] [PubMed] [Google Scholar]
- Prenafeta-Boldú FX, Summerbell R, Hoog GS de (2006). Fungi growing on aromatic hydrocarbons: biotechnology's unexpected encounter with biohazard? FEMS Microbiology Reviews 30: 109-130. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P (2000). Inference of population structure using multilocus genotype data. Genetics 155: 945-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard-Yegres N, Yegres F (1987). Cladosporium carrionii en vegetacion xerofila: aislamiento en una zona endemica para la cromomicosis en Venezuela. Dermatologia Venezuelana 25: 15-18. [Google Scholar]
- Richard-Yegres N, Yegres, Zeppenfeldt G (1992). Cromomicosis: endemia rural, laboral y familiar en Venezuela. Revista Iberoamericana Micologia 9: 38-41. [Google Scholar]
- Riddley M (1957). The natural habitat of Cladosporium carrionii a cause of chromoblastomycosis. Australian Journal of Dermatology 4: 23-27. [DOI] [PubMed] [Google Scholar]
- Rubin HA, Bruce S, Rosen T, McBride ME (1991). Evidence for percutaneous inoculation as the mode of transmission for chromoblastomycosis. Journal of the American Academy of Dermatology 25: 951-954. [DOI] [PubMed] [Google Scholar]
- Salgado L, Pereira da Silva J, Picanço Diniz JÁ, Batista da Silva M, Fagundes da Costa P, Teixeira C, Salgado UI (2004). Isolation of Fonsecaea pedrosoi from thorns of Mimosa pudica, a probable natural source of chromoblastomycosis. Revista Instituto de Medicina Tropical, Sao Paulo 46: 33-36. [DOI] [PubMed] [Google Scholar]
- Schoch CL, Shoemaker RA, Seifert KA, Hambleton A, Spatafora JW, Crous PW (2006). A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98: 1041-1052. [DOI] [PubMed] [Google Scholar]
- Seifert KA, Nickerson NL, Corlett M, Jackson ED, Louis-Seize G, Davies RJ (2004). Devriesia, a new hyphomycete genus to accommodate heat-resistant, cladosporium-like fungi. Canadian Journal of Botany 82: 914-926. [Google Scholar]
- Silva JP, Rocha RM da, Moreno JS (1995). The coconut babaçu (Orbignya phalerata) as a probable risk of human infection by the agent of chromoblastomycosis in the State of Maranhão, Brazil. Revista do Sociedad Brasiliera de Medicina Tropical, Sao Paulo 28: 49-52. [DOI] [PubMed] [Google Scholar]
- Swofford DL (1981). Utility of the distance-Wagner procedure. In: Advances in cladistics, vol. 1 (Funk VA and Brooks DR, eds.). Annals of the New York Botanical Gardens, New York: 25-44. [Google Scholar]
- Swofford DL (2003). PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sinauer Associates, Sunderland, Massachusetts.
- Szaniszlo PJ (2002). Molecular genetic studies of the model dematiaceous pathogen Wangiella dermatitidis. International Journal of Medical Microbiology 292: 381-390. [DOI] [PubMed] [Google Scholar]
- Tamura K, Nei M (1993). Estimation of the number of nucleotide substitutuions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biolology and Evolution 10: 512-526. [DOI] [PubMed] [Google Scholar]
- Trejos A (1953). Cromoblastomycosis experimental en Bufo marinus. Revista de Biologia Tropical 1: 39-53. [Google Scholar]
- Trejos A (1954). Cladosporium carrionii n. sp. and the problem of cladosporia isolated from chromoblastomycosis. Revista de Biologia Tropical 2: 75-112. [Google Scholar]
- Vélez F, Chávez J (1980). Los Cactus de Venezuela. Colecciones Inagro, Venezuela.
- White TJ, Bruns T, Lee S, Taylor J (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: a guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds). Academic Press, San Diego, California: 315-322.
- Yegres F, Niel F, Gantier JC, Richard-Yegres N (1998). Murine humoral immune response against Cladophialophora carrionii and Fonsecaea pedrosoi infection. Journal de Mycologie Médical 8: 179-182. [Google Scholar]
- Yegres F, Richard-Yegres N (2002). Cladophialophora carrionii: Aportes al conocimiento de la endemia en Venezuela durante el siglo XX. Revista de la Sociedad Venezuelana de Microbiología 2: 153-157. [Google Scholar]
- Yegres F, Richard-Yegres N, Medina-Ruiz E, González-Vivas R (1985). Cromomicosis por Cladosporium carrionii en criadores de caprinos del estado Falcon. Investigación Clínica 26: 235-246. [Google Scholar]
- Yegres F, Richard-Yegres N, Nishimura K, Miyaji M (1991). Virulence and pathogenicity of human and environmental isolates of Cladosporium carrionii in new born ddY mice. Mycopathologia 114: 71-76. [DOI] [PubMed] [Google Scholar]
- Yegres F, Richard-Yegres N, Perez-Blanco M (1996). Cromomicosis. In: Temas de Micologia Médica. (Bastardo de Albornoz M, ed.). Caracas, Venezuela: 87-102.
- Yegüez-Rodriguez J, Richard-Yegres N, Yegres F, Rodríguez Larralde A (1992). Cromomicosis: susceptibilidad genética en grupos familiares de la zona endémica en Venezuela. Acta Científica Venezuelana 43: 98-102. [Google Scholar]
- Zeng J-S, Sutton DA, Fothergill AW, Rinaldi MG, Harrak MJ, Hoog GS de (2007). Spectrum of clinically relevant Exophiala species in the U.S.A. Journal of Clinical Microbiology: in press. [DOI] [PMC free article] [PubMed]