Abstract
Background
A systematic review was undertaken to evaluate the efficacy of tiotropium, a long acting anticholinergic drug, on clinical events, symptom scales, pulmonary function, and adverse events in stable chronic obstructive pulmonary disease (COPD).
Methods
A systematic search was made of the Cochrane trials database, MEDLINE, EMBASE, CINAHL, and a hand search of 20 respiratory journals. Missing data were obtained from authors and the manufacturer. Randomised controlled trials of ⩾12 weeks' duration comparing tiotropium with placebo, ipratropium bromide, or long acting β2 agonists (LABA) were reviewed. Studies were pooled to yield odds ratios (OR) or weighted mean differences with 95% confidence intervals (CI).
Results
Nine trials (8002 patients) met the inclusion criteria. Tiotropium reduced the odds of a COPD exacerbation (OR 0.73; 95% CI 0.66 to 0.81) and related hospitalisation (OR 0.68; 95% CI 0.54 to 0.84) but not pulmonary (OR 0.50; 95% CI 0.19 to 1.29) or all‐cause (OR 0.96; 95% CI 0.63 to 1.47) mortality compared with placebo and ipratropium. Reductions in exacerbations and hospitalisations compared with LABA were not statistically significant. Similar patterns were evident for quality of life and symptom scales. Tiotropium yielded greater increases in forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) from baseline to 6–12 months than did placebo, ipratropium, and LABA. Decline in FEV1 over 1 year was 30 ml (95% CI 7 to 53) slower with tiotropium than with placebo and ipratropium (data were not available for LABA). Reports of dry mouth and urinary tract infections were increased with tiotropium.
Conclusions
Tiotropium reduced COPD exacerbations and related hospitalisations, improved quality of life and symptoms, and may have slowed the decline in FEV1. Long term trials are warranted to evaluate the effects of tiotropium on decline in FEV1 and to clarify its role compared with LABA.
Keywords: tiotropium, chronic obstructive pulmonary disease, emphysema, chronic bronchitis
Chronic obstructive pulmonary disease (COPD) is currently the fourth or fifth leading cause of death in the most developed countries, and is projected to be the third cause of death worldwide by 2020.1 Despite this burden, few pharmacological treatments for COPD have been proved to reduce clinical events, and none has been shown definitively to slow decline in forced expiratory volume in 1 second (FEV1).
Tiotropium has a quaternary ammonium structure related to that of ipratropium bromide. It dissociates slowly from M1 and M3 receptors but rapidly from M2 receptors,2 which allows once daily dosing and has theoretical advantages since M2 receptors are feedback inhibitory receptors.3,4
A number of randomised clinical trials suggest that tiotropium might reduce clinical event rates and improve lung function, but these trials have been of borderline statistical power. We therefore undertook a meta‐analysis of available randomised trials to evaluate the efficacy of tiotropium on clinical events, health related quality of life, symptoms, pulmonary function, and adverse events compared with placebo, ipratropium bromide, and long acting β2 agonists (LABA). An earlier version of this meta‐analysis was published electronically in the Cochrane Library.5
Methods
Data sources
The Cochrane Airways Review Group Specialised Register of COPD trials is a compilation of references to reports of controlled clinical trials assembled from systematic searches of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and CINAHL, and supplemented by hand searching of leading respiratory journals and conference abstracts. It is not limited by language of publication. The Register was searched using the following terms: tiotropium OR “Ba 679 BR” OR Spiriva OR oxitropium. In addition, a search of LILACS and CENTRAL was performed. Searches were current as of May 2006.
Reference lists of all primary studies and review articles were reviewed for additional references. Authors of identified randomised trials were asked about published and unpublished studies. The manufacturer of tiotropium (Boehringer Ingelheim) was contacted regarding overlap between studies, unpublished studies, and supplemental data. Additional data were obtained from the Food and Drug Administration website.6
Study selection
The following criteria were used to select randomised controlled trials for inclusion in the meta‐analysis:
Target population: stable COPD consistent with American Thoracic Society (ATS)/European Respiratory Society (ERS) criteria,7 without evidence of an exacerbation for 1 month prior to study entry;
Intervention: randomised clinical trials comparing tiotropium with placebo, ipratropium bromide, or LABA;
Methodological criteria: studies that followed patients for 12 weeks or more after randomisation.
Two reviewers independently identified trials that appeared potentially relevant from titles and abstracts. Using the abstract or the full text of each study, as necessary, two reviewers independently decided if trials fulfilled inclusion criteria for the review. Differences were resolved by discussion.
Data extraction and assessment of methodological quality
Two reviewers independently extracted data. Intention‐to‐treat results were used whenever available. Primary clinical outcomes were COPD exacerbations, related hospitalisations, and all‐cause mortality. Secondary outcomes included disease specific mortality, health related quality of life scales (St George's Respiratory Questionnaire [SGRQ]8), symptom scores (Transitional Dyspnea Index [TDI], a multidimensional measure of breathlessness9), change in trough FEV1 and forced ventilatory capacity (FVC) from baseline and from steady state 8–15 days after randomisation, and adverse events (dry mouth, constipation, urinary infection and obstruction, chest pain, myocardial infarction, arrhythmias and congestive heart failure). Methodological quality was assessed using the Cochrane approach and Jadad criteria.10
Statistical analysis
Trials were combined using RevMan (Version 4.2.8). Fixed effect odds ratios (OR) for dichotomous variables and weighted mean differences (WMD) for continuous variables with 95% confidence intervals (CI) were calculated for individual trials. Trials were pooled using fixed effect OR or WMD as appropriate. Heterogeneity was tested using the Breslow‐Day test with a p value <0.1 considered statistically significant. A random effects model was used if heterogeneity was found. Weighted averages of cumulative incidences in the control groups were calculated across all trials and for trials of 12 months' duration. Numbers needed to treat (NNT) were calculated from the pooled OR, 95% CI, and cumulative incidences in the control groups of the 12 month trials.11
For each outcome, trials were pooled within categories of control group (placebo, ipratropium, or LABA). Since an earlier large randomised clinical trial showed that ipratropium does not reduce clinical events or slow the decline in FEV1 relative to placebo,12,13 summary estimates were calculated comparing tiotropium with placebo or ipratropium for these end points when there was statistical homogeneity across categories of control group. Adverse events were combined across all categories of control group when there was statistical homogeneity.
Publication bias was examined in funnel plots and tested with a modified Macaskill's test.14 The effects of tiotropium were examined across predefined subgroups by disease severity and concurrent LABA use.
Results
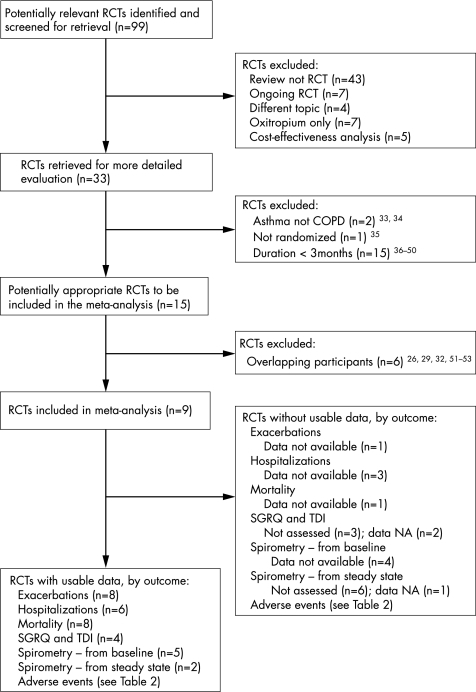
Ninety nine articles were identified, of which 33 possibly fulfilled the inclusion criteria and 15 met the inclusion criteria (fig 1). Three of these articles reported the combined results of pairs of previously published and unpublished trials, and three others were secondary reports with overlapping participants. The net number of included trials was nine (8002 randomised patients). Table 1 shows the characteristics of the nine included trials.
Figure 1 QUOROM flow diagram.
Table 1 Characteristics of the double blind randomised clinical trials included in the analysis.
| Study | Duration of trial/no randomised/ pre‐randomisation run‐in | Inclusion criteria/exclusion criteria/participant characteristics | Permitted co‐therapies/discontinued co‐therapies (% on co‐therapy at baseline) | Control group intervention(s) | |||
|---|---|---|---|---|---|---|---|
| Beeh20 | 12 weeks N = 1639 1 week washout period | Inclusion: COPD, FEV1 ⩽70% predicted, ratio ⩽70%, age >40 years, smoking history >10 py | Permitted: SABA (76%), inhaled corticosteroid (57%), prednisone (16%), theophylline (52%)Discontinued: ipratropium (69%), LABA (50%) | Placebo | |||
| Exclusion: asthma, allergic rhinitis, atopy, oxygen use, arrhythmia, recent MI or CHF hospitalisation | |||||||
| Characteristics: Mean age 62 years; 75% male; FEV1: 1.3 (0.5) l; FVC 2.4 (0.7) l; ratio NA | |||||||
| Brusasco17 | 6 months N = 1207 2 week washout period | Inclusion: COPD, FEV1 ⩽65% predicted, ratio ⩽70%, age >40 years, smoking history >10 pyExclusion: asthma, allergic rhinitis, atopy, total eosinophil count ⩾600/mm3, oxygen use, URI <6 weeks, other significant diseaseCharacteristics: Mean age 64 years; 76% male; FEV1 1.1 (0.4) l; FVC 2.6 (0.7) l; ratio 43 (10)% | Permitted: NA (Donohue30 lists SABA (66%), inhaled corticosteroid (66%), prednisone (6%), theophylline (21%)) | (1) Salmeterol 50 μg bid by MDI (2) Placebo | |||
| Discontinued: NA (Donohue30 lists ipratropium (53%), LABA (NA)) | |||||||
| Briggs16 | 12 weeks N = 653 2 week washout period | Inclusion: COPD, FEV1 ⩽60% predicted, ratio ⩽70%, age >40 years, smoking history >10 py | Permitted: SABA (58%), inhaled corticosteroid (50%), prednisone (2%)Discontinued: ipratropium (55%), LABA (47%), theophylline (12%) | Salmeterol 50 μg bid by MDI | |||
| Exclusion: asthma, allergic rhinitis, atopy, total eosinophil count ⩾600/mm3, renal insufficiency, prostatic hypertrophy, glaucoma, other significant disease, COPD exacerbation <4 weeks, prednisone ⩾10 mg/day, β blockers, oxygen use, recent pulmonary rehabilitation | |||||||
| Characteristics: Mean age 64 years; 66% male; FEV1 1.0 (0.4) l; FVC 2.4 (0.7) l; ratio 43 (10)% | |||||||
| Casaburi19 | 12 months N = 921 2 week washout period | Inclusion: COPD, FEV1 ⩽65% predicted, ratio ⩽70%, age ⩾40 years, smoking history >10 pyExclusion criteria: asthma, allergic rhinitis, atopy, total eosinophil count ⩾600/mm3, oxygen use, prednisone ⩾10 mg in prior month, MI <1 year, CHF <3 years, arrhythmiaCharacteristics: Mean age 65 years; 65% male; FEV1 1.0 (0.4) l; FVC 2.3 (0.8) l; ratio 46 (12)% | Permitted: SABA (99%), inhaled corticosteroid (42%), prednisone (7%), theophylline (23%) | Placebo | |||
| Discontinued: ipratropium (57%), LABA (NA) | |||||||
| Casaburi30 | 25 weeks N = 108 1 week training run‐in | Inclusion: COPD, FEV1 ⩽60% predicted, ratio ⩽70%, age ⩾40 years, smoking history >10 py, able to perform pulmonary rehabilitation | Permitted: SABA, inhaled and prednisone, theophylline (% NA)Discontinued: ipratropium, LABA (% NA) | Placebo | |||
| Exclusion: asthma, allergic rhinitis, atopy, total eosinophil count ⩾600/mm3, BMI <18 or >30 kg/m2, other significant disease, recent URI, MI, CHF, arrhythmia | |||||||
| Characteristics: Mean age 67 years; 56% male; FEV1 0.9 (0.4) l; FVC% 34 (12); ratio 43 (11)% | |||||||
| Dusser21 | 48 weeks N = 1050 3 week run‐in | Inclusion: COPD, pre‐BD FEV1 30‐65% predicted, FEV1/SVC ⩽70%, age >40 years, smoking history >10 py, ⩾1 exacerbation in prior year | Permitted: SABA (94%), inhaled corticosteroid (63%), prednisone (2%)Discontinued: ipratropium (38%), LABA (32%), theophylline (7%) | Placebo | |||
| Exclusion: asthma, allergic rhinitis, atopy, renal insufficiency, oxygen use, COPD exacerbation<6 weeks, prednisone ⩾10 mg/day, other significant medical illness | |||||||
| Characteristics: Mean age: 65 years; 88% male; FEV1 1.4 (0.4) l; FVC 2.6 (0.8) l; ratio 55 (12)% | |||||||
| Niewoehner18 | 6 months N = 1829 No run‐in period | Inclusion: COPD, FEV1 ⩽60% predicted, ratio ⩽70%, age >40 years, smoking history >10 pyExclusion: asthma, renal insufficiency, prostatic hypertrophy, glaucoma, MI <6 months, arrhythmia, CHF hospitalisation <1 year, on cancer treatment, COPD exacerbation <4 weeks, prednisone ⩾20 mg/day | Permitted: SABA (94%), LABA (38%), inhaled corticosteroid (58%), prednisone (10%), theophylline (14%), oxygen (29%)Discontinued: ipratropium (80%) | Placebo | |||
| Characteristics: Mean age 68 years; 99% male; FEV1 1.0 (0.4) l; ratio 48 (11)% | |||||||
| Verkindre31 | 12 weeks N = 100 2 weeks run‐in | Inclusion: COPD, FEV1 ⩽50% predicted, FEV1/SVC ⩽70% predicted, RV ⩽125% predicted, age >40 years, smoking history >10 py, ⩾1 exacerbation in previous year | Permitted: SABA, inhaled and prednisone, theophylline (% NA)Discontinued: ipratropium, LABA (% NA) | Placebo | |||
| Exclusion: asthma, allergic rhinitis, atopy, total eosinophil count ⩾600/mm3, MI <1 year, arrhythmia, CHF <3 years, oxygen use, COPD exacerbation <6 weeks, prednisone ⩾10 mg/day | |||||||
| Characteristics: Mean age 59 years; 94% male; FEV1 1.1 (0.3) l; FVC 2.4 (0.7) l; ratio 40 (7)% | |||||||
| Vincken15 | 12 months N = 535 2 week washout period | Inclusion: COPD, FEV1 ⩽65% predicted, ratio ⩽70%, age ⩾40 years, smoking history >10 pyExclusion: asthma, allergic rhinitis, atopy, total eosinophil count ⩾600/mm3, oxygen use, recent URI, other significant disease (van Noord32 lists MI <1 year, CHF <3 years, arrhythmia, prostatic hypertrophy, glaucoma, anticholinergic drug allergy) | Permitted: SABA (76%), inhaled corticosteroid (80%), prednisone (9%), theophylline (16%)Discontinued: ipratropium (60%), LABA (NA) | Ipratropium 40 μg qid by MDI | |||
| Characteristics: Mean age 64 years; 85% male; FEV1 1.2 (0.4) l; FVC 2.7 (0.8); ratio 46 (10)% | |||||||
py, pack years; MI, myocardial infarction; CHF, congestive heart failure; NA, not available; URI, upper respiratory infection; SABA, short acting bronchodilator; LABA, long acting bronchodilator; MDI, metered dose inhaler.
Six of the included trials compared tiotropium with placebo, one compared tiotropium with ipratropium,15 one compared tiotropium with a LABA (salmeterol),16 and one compared tiotropium with placebo and with salmeterol.17 Six trials scored four out of five for methodological quality, two scored five out of five,15,18 and one scored three out of five.19 Allocation concealment was described in only one trial.15 The protocols were extremely similar. All trials enrolled patients regardless of response to bronchodilators but excluded patients with a prior history of asthma; all but one18 excluded patients with a history of atopy or allergic rhinitis; and six excluded patients with a raised eosinophil count. All trials prohibited the use of non‐study ipratropium and all but one18 prohibited the use of non‐study LABA.
The weighted mean duration of the trials was 7.0 months (range 3–12). The severity of COPD was generally moderate to severe (ERS/ATS stage III–IV; range stage II–V); 38–80% of patients were taking ipratropium at enrolment, 32–50% were taking LABA, and 42–80% were taking inhaled corticosteroids.
Clinical events
COPD exacerbations
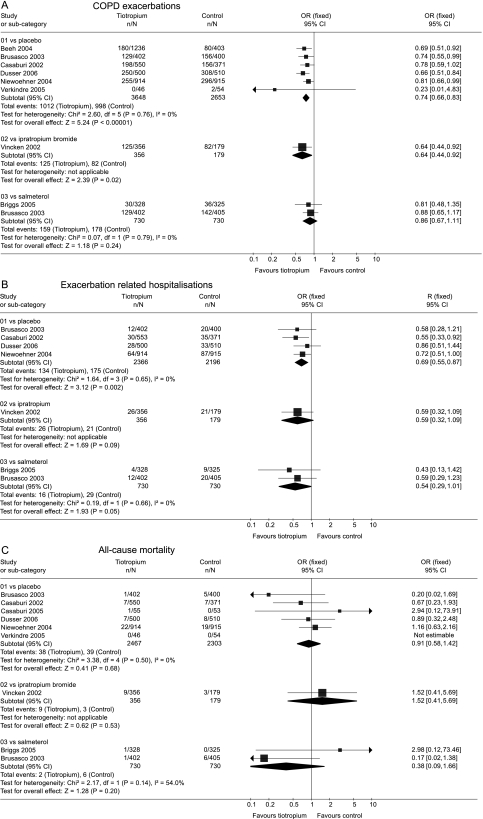
The cumulative incidence of COPD exacerbations among controls was 35% over the mean duration (7.0 months) of all trials, and 52% in the 1 year trials. Tiotropium reduced COPD exacerbations compared with placebo and compared with ipratropium (fig 2A). The cumulative incidence of exacerbations was lower with tiotropium than with salmeterol, but this difference was smaller and not statistically significant. The treatment effect of tiotropium was statistically homogeneous across the control groups (p = 0.77) and the summary OR for tiotropium compared with placebo or ipratropium was 0.73 (95% CI 0.66 to 0.81). The corresponding NNT for tiotropium to prevent one exacerbation per year was 13 (95% CI 10 to 21).
Figure 2 Summary effects of tiotropium on (A) COPD exacerbations, (B) hospitalisations, and (C) all‐cause mortality.
Hospitalisations for COPD exacerbations
The cumulative incidence of exacerbation related hospitalisations among controls was 7% over the duration of all trials, and 9% in the 1 year trials. Tiotropium reduced the risk of hospitalisation for COPD exacerbations compared with placebo (fig 2B). Similar reductions in hospitalisations were observed compared with ipratropium and compared with salmeterol, but neither of these differences was statistically significant. The treatment effect of tiotropium was statistically homogeneous across the control groups (p = 0.76) and the summary estimate for tiotropium compared with placebo or ipratropium was OR 0.68 (95% CI 0.54 to 0.84). The corresponding NNT for tiotropium to prevent one exacerbation related hospitalisation per year was 38 (95% CI 26 to 76).
Mortality
Cumulative all‐cause mortality among controls was 1.5% over the duration of all trials and 1.7% in the 1 year trials. There were no statistically significant differences in all‐cause mortality between tiotropium and placebo, ipratropium, or salmeterol (fig 2C). The trials were statistically homogeneous across the control groups (p = 0.57) and the summary estimate for tiotropium compared with placebo or ipratropium was not significant (OR 0.96; 95% CI 0.63 to 1.47).
Mortality from pulmonary causes was non‐significantly lower with tiotropium compared with placebo or ipratropium (OR 0.50; 95% CI 0.19 to 1.29; fig S1 available online only at http://www.thoraxjnl.com/supplemental). Heterogeneity was not evident. There were no statistically significant differences for cardiovascular mortality (OR 1.17; 95% CI 0.54 to 2.51), cancer mortality (0.77; 95% CI 0.28 to 2.12), and mortality from other causes (OR 2.77; 95% CI 0.81 to 9.45).
Health related quality of life and symptom scales
St George's Respiratory Questionnaire (SGRQ)
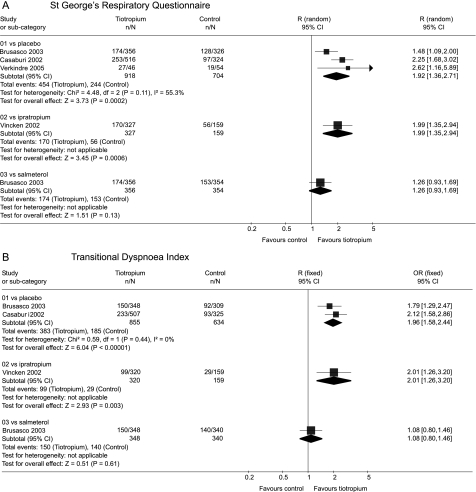
The mean change in SGRQ over the course of the trials was larger with tiotropium than with placebo (WMD −3.3; 95% CI −4.6 to −2.0) or with ipratropium (WMD −3.3; 95% CI −5.6 to −1.0). A smaller and non‐significant difference was observed compared with salmeterol (WMD −1.4; 95% CI −3.2 to 0.4). The trials were statistically homogeneous across the control groups (p = 0.31) and the summary estimate for tiotropium compared with placebo or ipratropium was an improvement of WMD −3.3 (95% CI −4.7 to −2.2).
Similar results were observed for the proportion with a clinically significant change in SGRQ (fig 3A), although there was evidence of heterogeneity across the control groups (p = 0.04).
Figure 3 Summary effects of tiotropium on clinically significant changes in (A) St George's Respiratory Questionnaire and (B) Transitional Dyspnoea Index.
Transitional Dyspnoea Index (TDI)
Data on mean change in TDI was inadequate for meta‐analysis. The results for the proportion with a clinically significant change in TDI (fig 3B) were similar to those for SGRQ. There was evidence of heterogeneity across the control groups (p = 0.07).
Spirometric indices
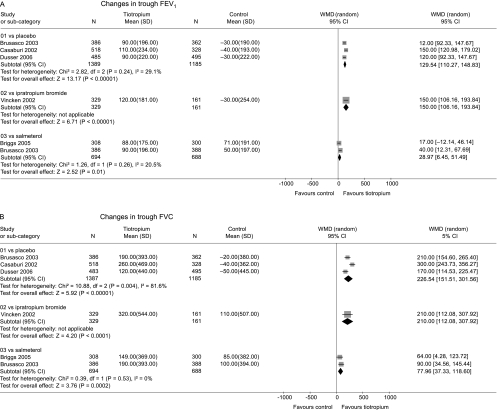
Change in FEV1 and FVC from baseline
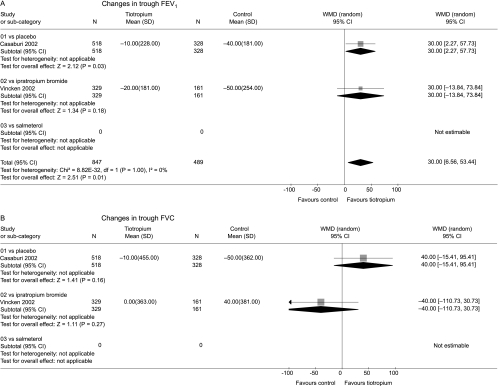
The mean improvement in trough FEV1 from baseline to the end of the trials was greater with tiotropium than with placebo or ipratropium (fig 4A). A smaller but statistically significant difference was observed compared with salmeterol. There was evidence of statistical heterogeneity across the control groups (p<0.0001) which arose from the smaller mean difference compared with salmeterol. Similar results were seen for change in trough FVC from baseline (fig 4B).
Figure 4 Summary effects of tiotropium on changes in (A) trough FEV1 and (B) trough FVC from baseline before randomisation until end of trials.
Change in FEV1 and FVC from steady state
The mean decline in trough FEV1 from steady state was slower with tiotropium than with placebo (fig 5A). The treatment effect of tiotropium was similar to that of ipratropium, although the latter result was not statistically significant. The trials were statistically homogeneous across the control groups (p>0.99) and the summary estimate showed a WMD of 30 ml (95% CI 7 to 53 ml) slower decline in FEV1 for tiotropium compared with placebo or ipratropium.
Figure 5 Summary effects of tiotropium on changes in (A) trough FEV1 and (B) trough FVC from steady state 8 days after randomisation until end of trials (1 year).
Declines in trough FVC from steady state to the end of the two trials were heterogeneous (p = 0.08) and no statistically significant differences were observed between tiotropium and either control group (fig 5B).
Adverse events
Available data on adverse events are summarised in table 2. Dry mouth was significantly increased with tiotropium compared with placebo, ipratropium and salmeterol, and urinary tract infections were significantly increased compared with placebo and ipratropium (data were not available for salmeterol). Consistent but not statistically significant increases were observed for systemic anticholinergic adverse events (constipation and urinary retention). Heterogeneity was evident for arrhythmias or atrial fibrillation overall and in comparison with placebo (p = 0.05). This heterogeneity resulted from one trial that reported atrial fibrillation results only. When this trial was excluded, heterogeneity was not evident (p = 0.71) and the frequency of arrhythmias was significantly higher with tiotropium than with placebo (OR 2.33; 95% CI 1.11 to 4.88).
Table 2 Adverse events with tiotropium compared with placebo, ipratropium, and salmeterol, with summary estimates across all available data.
| Tiotropium compared with | p value for heterogeneity | Summary estimate | |||
|---|---|---|---|---|---|
| Placebo | Ipratropium | Salmeterol | |||
| Dry mouth | |||||
| Trials | 4 | 1 | 2 | 0.24 | 7 |
| Participants | 2835 | 535 | 1460 | 4830 | |
| Odds ratio | 4.6 | 2.1 | 4.7 | 3.9 | |
| (95% CI) | (3.0 to 7.1) | (1.05 to 4.2) | (2.4 to 9.2) | (2.8 to 5.5) | |
| Constipation | |||||
| Trials | 2 | 1 | 0 | 0.41 | 3 |
| Participants | 1931 | 535 | 2466 | ||
| Odds ratio | 2.2 | 0.5 | 1.7 | ||
| (95% CI) | (0.95 to 4.8) | (0.1 to 3.6) | (0.8 to 3.7) | ||
| Urinary retention | |||||
| Trials | 3 | 0 | 1 | 0.85 | 4 |
| Participants | 2733 | 807 | 3540 | ||
| Odds ratio | 2.5 | 3.0 | 2.6 | ||
| (95% CI) | (0.5 to 14) | (0.1 to 75) | (0.6 to 12) | ||
| Urinary tract infection | |||||
| Trials | 3 | 1 | 0 | 0.91 | 4 |
| Participants | 2733 | 535 | 3268 | ||
| Odds ratio | 1.6 | 1.8 | 1.6 | ||
| (95% CI) | (0.97 to 2.6) | (0.6 to 5.5) | (1.03 to 2.6) | ||
| Chest pain | |||||
| Trials | 3 | 1 | 1 | 0.09 | – |
| Participants | 2733 | 535 | 807 | ||
| Odds ratio | 0.9 | 2.5 | 1.2 | ||
| (95% CI) | (0.4 to 2.0) | (0.8 to 7.4) | (0.6 to 2.4) | ||
| Myocardial infarction | |||||
| Trials | 3 | 1 | 0 | 0.77 | 4 |
| Participants | 2733 | 535 | 3268 | ||
| Odds ratio | 1.0 | 1.5 | 1.1 | ||
| (95% CI) | (0.2 to 3.9) | (0.2 to 15) | (0.3 to 3.6) | ||
| Arrhythmia or atrial fibrillation | |||||
| Trials | 4 | 1 | 0 | 0.05 | – |
| Participants | 4561 | 535 | |||
| Odds ratio | 1.4 | 0.8 | |||
| (95% CI) | (0.4 to 5.7) | (0.3 to 1.8) | |||
| Congestive heart failure | |||||
| Trials | 3 | 1 | 0 | 0.86 | 4 |
| Participants | 2837 | 535 | 3372 | ||
| Odds ratio | 0.8 | 0.5 | 0.8 | ||
| (95% CI) | (0.4 to 1.6) | (0.1 to 8.1) | (0.4 to 1.5) | ||
Subgroup and sensitivity analyses
The trials were very similar with respect to disease severity and concurrent LABA use. The two trials with the highest baseline mean FEV120,21 had a statistically similar estimate for exacerbations as the pooled estimate and as a trial in which 29% of patients were on oxygen18 (fig 2).
The effect of tiotropium on exacerbations in the one trial18 that permitted concurrent use of LABA (OR 0.81; 95% CI 0.66 to 0.99) was statistically similar to the others that withheld LABA (OR 0.70; 95% CI 0.62 to 0.80).
Sensitivity analyses by quality weighting and random effects models yielded nearly identical results. Funnel plots for the primary end points showed no clear evidence of publication bias and the modified Macaskill test did not suggest publication bias for exacerbations (p = 0.65).
Discussion
This systemic review of the currently available randomised trials of tiotropium for stable COPD showed that tiotropium reduced COPD exacerbations and related hospitalisations compared with placebo or ipratropium. Increases in FEV1 and FVC from baseline were significantly larger with tiotropium than with placebo, ipratropium, and LABA. The decline in trough FEV1 from steady state was slower with tiotropium than with placebo or ipratropium, and pulmonary mortality was non‐significantly lower with tiotropium.
The benefits observed with tiotropium for exacerbations and related hospitalisations were large and clinically important. Consistent with these findings, tiotropium has been shown to be cost effective although not cost saving compared with ipratropium in Europe.22 The magnitude of the reduction in exacerbation related hospitalisations with tiotropium was similar in comparison with placebo, ipratropium and salmeterol, and was similar in large placebo controlled trials that did and did not permit use of LABA.
Changes in health related quality of life, symptom scales, and spirometric indices also appeared clinically significant. Compared with placebo and ipratropium, the mean change in the SGRQ across all participants was close to the clinically significant change in SGRQ of 4 units, and more participants on tiotropium achieved a clinically significant change in SQRQ and TDI compared with placebo and ipratropium. Improvements in spirometric indices from baseline were clinically significant compared with placebo and ipratropium at a threshold for FEV1 of 100 ml23 but not at a threshold of 225 ml.24 Improvements in spirometric indices from baseline were statistically but not clinically significant compared with salmeterol.
The results of this systemic review are consistent with a previous review of treatments for COPD25 which reported on exacerbations and quality of life but which was limited by double counting of patients randomised to tiotropium. Our results correct and extend that review with more than twice the number of randomised patients and additional outcomes of hospitalisations, mortality, symptom scales, spirometric indices, and adverse events.
We found that the decline in trough FEV1 from steady state was slower with tiotropium than with placebo or ipratropium. This difference was large relative to the difference observed in a meta‐analysis of inhaled corticosteroids in COPD26 and was consistent with a post hoc analysis of one of the tiotropium trials.27 However, this observation should be interpreted with caution as it might be due to (1) incomplete attainment of steady state of tiotropium at 8 days; (2) chance, given that multiple spirometric indices were measured and that the duration of the relevant trials was only 1 year; and (3) bias, given that most but possibly not all trial results for this measure were available for meta‐analysis. Larger longer term trials are necessary to assess the validity of this result, which would be of major clinical relevance if replicated.
Mortality from pulmonary causes was non‐significantly lower among those randomised to tiotropium compared with placebo or ipratropium. This finding suggests that observed benefits on exacerbations and hospitalisations might translate into reductions in pulmonary mortality, but requires evaluation in long term randomised trials designed specifically to examine pulmonary mortality. Estimates for disease‐specific mortality can be subject to more biases than all‐cause mortality, and we note that all‐cause mortality did not differ appreciably between tiotropium and placebo.
The trials included in this review were of good quality and used almost identical designs with regard to inclusion and exclusion criteria. The clinical homogeneity of the trials resulted in statistical homogeneity for most outcome measures across the trials. We calculated summary estimates of the effects of tiotropium compared with placebo and ipratropium. Heterogeneity would be introduced if ipratropium had an effect on the relevant outcomes, but ipratropium has been shown not to alter the long term decline in FEV1,13 hospitalisations or survival12 compared with placebo. LABA, on the other hand, may reduce exacerbations compared with placebo.25,28
Potential limitations of meta‐analyses include double counting of patients from overlapping publications, publication bias, reporting bias, and selection bias from differential inclusion of available trials. We avoided double counting by discussing trial overlap with the primary authors and the manufacturer of tiotropium, and evaluated for publication bias with funnel plots and statistical tests. Selective reporting of secondary end points and of non‐intention to treat reports in published manuscripts may bias results; we minimised this bias by obtaining supplemental data for five of the nine included studies, although complete intention to treat analyses were missing for most studies due to missing data. We avoided selection bias by pre‐specified inclusion and exclusion criteria, a systematic search, and independent evaluation of trial inclusion by two reviewers.
In conclusion, tiotropium reduced COPD exacerbations and exacerbation related hospitalisations compared with placebo or ipratropium. It also improved health related quality of life and symptom scores and can be recommended for the treatment of stable COPD. The results of this systematic review suggest that tiotropium may slow the decline in FEV1, although this finding requires confirmation in additional long term randomised clinical trials.
Figure S1 showing mortality from pulmonary causes, cardiovascular causes, cancer and other causes is available online at http://www.thoraxjnl.com/ supplemental.
Copyright © 2006 BMJ Publishing Group and British Thoracic Society
Supplementary Material
Acknowledgements
The authors thank Maria Martinez‐Torres for assistance with manuscript preparation and various individuals at Boehringer‐Ingelheim who helped provide unpublished data to strengthen this systematic review. The assistance of Phillippa Poole (Cochrane Airways Review Group co‐editor) was greatly appreciated.
Abbreviations
COPD - chronic obstructive pulmonary disease
FEV1 - forced expiratory volume in 1 second
FVC - forced vital capacity
LABA - long acting β2 agonist
SGRQ - St George's Respiratory Questionnaire
TDI - Transitional Dyspnoea Index
Footnotes
Funding: Robert Wood Johnson Generalist Physician Faculty Scholar Award and National Institutes of Health (USA) HL075476, HL077612, HL063841.
Competing interests: Dr Barr: none. Dr Bourbeau has received honoraria for CME, membership on advisory boards and financial support from government agencies, contract and investigator initiated research studies for a number of companies including Altana, Astra Zeneca, Bayer, Boehringer‐Ingelheim, GlaxoSmithKline, Novartis and Pfizer. Dr Camargo has received investigator initiated grants and consulting/lecture honoraria from AstraZeneca, Boehringer‐Ingelheim, GlaxoSmithKline, and Novartis. Dr Ram: none.
Figure S1 showing mortality from pulmonary causes, cardiovascular causes, cancer and other causes is available online at http://www.thoraxjnl.com/ supplemental.
References
- 1.Murray C J L, Lopez A D. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 19973491498–1504. [DOI] [PubMed] [Google Scholar]
- 2.Haddad E, Mak J, Barnes P. Characterization of 3H Ba 679 BR, a slowly dissociation muscarinic antagonist, in human lung: radioligand binding and autoradiographic mapping. Mol Pharmacol 199445899–907. [PubMed] [Google Scholar]
- 3.Disse B, Speck G A, Rominger K L.et al Tiotropium (Spiriva): mechanistical considerations and clinical profile in obstructive lung disease. Life Sci 199964457–464. [DOI] [PubMed] [Google Scholar]
- 4.Barnes P J. The pharmacological properties of tiotropium. Chest 2000117(2 Suppl)63–6S. [DOI] [PubMed] [Google Scholar]
- 5.Barr R G, Bourbeau J, Camargo C A., Jret al Inhaled tiotropium for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews, 2005; Issue 2 [DOI] [PMC free article] [PubMed]
- 6.Food and Drug Administration, Pulmonary‐Allergy Drugs Advisory Committee Clinical briefing document. Integrated review of safety. NDA 21‐395. Food and Drug Administration 2002
- 7.Celli B R, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 200423932–946. [DOI] [PubMed] [Google Scholar]
- 8.Jones P W, Quirk F H, Baveystock C M.et al A self‐complete measure of health status for chronic airflow limitation. The St George's Respiratory Questionnaire. Am Rev Respir Dis 19921451321–1327. [DOI] [PubMed] [Google Scholar]
- 9.Mahler D A, Weinberg D H, Wells C K.et al The measurement of dyspnea: contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest 198485751–758. [DOI] [PubMed] [Google Scholar]
- 10.Jadad A R, Moore R A, Carroll D.et al Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996171–12. [DOI] [PubMed] [Google Scholar]
- 11.Cates C. Visual Rx. www.nntonline.net, 2006
- 12.Anthonisen N R, Connett J E, Enright P L.et al Hospitalizations and mortality in the Lung Health Study. Am J Respir Crit Care Med 2002166333–339. [DOI] [PubMed] [Google Scholar]
- 13.Anthonisen N R, Connett J E, Kiley J P.et al Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA 19942721497–1505. [PubMed] [Google Scholar]
- 14.Macaskill P, Walter S D, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Stat Med 200120641–654. [DOI] [PubMed] [Google Scholar]
- 15.Vincken W, van Noord J A, Greefhorst A P.et al Dutch/Belgium Tiotropium Study Group. Improved health outcomes in patients with COPD during one year's treatment with tiotropium. Eur Respir J 200219209–216. [DOI] [PubMed] [Google Scholar]
- 16.Briggs D D, Jr, Covelli H, Lapidus R.et al Improved daytime spirometric efficacy of tiotropium compared with salmeterol in patients with COPD. Pulm Pharmacol Ther 200518397–404. [DOI] [PubMed] [Google Scholar]
- 17.Brusasco V, Hodder R, Miravitlles M.et al Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax 200358399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niewoehner D, Rice K, Cote C.et al Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once‐daily inhaled anticholinergic bronchodilator. A randomized trial. Ann Intern Med 2005143317–326. [DOI] [PubMed] [Google Scholar]
- 19.Casaburi R, Mahler D A, Jones P W.et al A long‐term evaluation of once‐daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J 200219217–224. [DOI] [PubMed] [Google Scholar]
- 20.Beeh K M, Beier J, Buhl R.et al Efficacy of tiotropium bromide (Spiriva) in patients with chronic obstructive pulmonary disease (COPD) of different severities (in German). Pneumologie 200660341–346. [DOI] [PubMed] [Google Scholar]
- 21.Dusser D, Bravo M ‐ L, Iacono P, on behalf of the MISTRAL Study Group The effect of tiotropium on exacerbations and airflow in patients with COPD. Eur Respir J 200627547–555. [DOI] [PubMed] [Google Scholar]
- 22.Oostenbrink J B, Rutten‐van Molken M P, Al M J.et al One‐year cost‐effectiveness of tiotropium versus ipratropium to treat chronic obstructive pulmonary disease. Eur Respir J 200423241–249. [DOI] [PubMed] [Google Scholar]
- 23.Redelmeier D A, Goldstein R S, Min S T.et al Spirometry and dyspnea in patients with COPD. When small differences mean little. Chest 19961091163–1168. [DOI] [PubMed] [Google Scholar]
- 24.Herpel L B, Kanner R E, Lee S M.et al Variability of spirometry in chronic obstructive pulmonary disease: results from two clinical trials. Am J Respir Crit Care Med 20061731106–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sin D D, McAlister F A, Anthonisen N R.et al Contemporary management of chronic obstructive pulmonary disease: scientific review. JAMA 20032902301–2312. [DOI] [PubMed] [Google Scholar]
- 26.Sutherland E R, Allmers H, Ayas N T.et al Inhaled corticosteroids reduce the progression of airflow limitation in chronic obstructive pulmonary disease: a meta‐analysis. Thorax 200358937–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anzueto A, Tashkin D, Menjoge S.et al One‐year analysis of longitudinal changes in spirometry in patients with COPD receiving tiotropium. Pulm Pharmacol Ther 20051875–81. [DOI] [PubMed] [Google Scholar]
- 28.Mahler D A, Donohue J F, Barbee R A.et al Efficacy of salmeterol xinafoate in the treatment of COPD. Chest 1999115957–965. [DOI] [PubMed] [Google Scholar]
- 29.Donohue J F, van Noord J A, Bateman E D.et al A 6‐month, placebo‐controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest 200212247–55. [DOI] [PubMed] [Google Scholar]
- 30.Casaburi R, Kukafka D, Cooper C B.et al Improvement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPD. Chest 2005127809–817. [DOI] [PubMed] [Google Scholar]
- 31.Verkindre C, Bart F, Aquilaniu B.et al The effect of tiotropium on hyperinflation and exercise capacity in chronic obstructive pulmonary disease. Respiration 200673420–427. [DOI] [PubMed] [Google Scholar]
- 32.van Noord J A, Bantje T A, Eland M E.et al A randomised controlled comparison of tiotropium and ipratropium in the treatment of chronic obstructive pulmonary disease. The Dutch Tiotropium Study Group. Thorax 200055289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Connor B J, Towse L J, Barnes P J. Prolonged effect of tiotropium bromide on methacholine‐induced bronchoconstriction in asthma. Am J Respir Crit Care Med 1996154876–880. [DOI] [PubMed] [Google Scholar]
- 34.Terzano C, Petroianni A, Ricci A.et al Early protective effects of tiotropium bromide in patients with airways hyperresponsiveness. Eur Rev Med Pharmacol Sci 20048259–264. [PubMed] [Google Scholar]
- 35.Maesen F P, Smeets J J, Costongs M A.et al Ba 679 Br, a new long‐acting antimuscarinic bronchodilator: a pilot dose‐escalation study in COPD. Eur Respir J 199361031–1036. [PubMed] [Google Scholar]
- 36.Maesen F P V, Smeets J J, Sledsens T J.et al Tiotropium bromide, a new long‐acting anti‐muscarinic bronchodilator: a pharmacodynamic study in patients with chronic obstructive pulmonary disease (COPD). Dutch Study Group. Eur Respir J 199581506–1513. [PubMed] [Google Scholar]
- 37.Littner M R, Ilowite J S, Tashkin D P.et al Long‐acting bronchodilation with once‐daily dosing of tiotropium (Spiriva) in stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 20001611136–1142. [DOI] [PubMed] [Google Scholar]
- 38.van Noord J A, Smeets J J, Custers F L.et al Pharmacodynamic steady state of tiotropium in patients with chronic obstructive pulmonary disease. Eur Respir J 200219639–644. [DOI] [PubMed] [Google Scholar]
- 39.Celli B, ZuWallack R, Wang S.et al Improvement in resting inspiratory capacity and hyperinflation with tiotropium in COPD patients with increased static lung volumes. Chest 20031241743–1748. [DOI] [PubMed] [Google Scholar]
- 40.Calverley P M, Lee A, Towse L.et al Effect of tiotropium bromide on circadian variation in airflow limitation in chronic obstructive pulmonary disease. Thorax 200358855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cazzola M, Di Marco F, Santus P.et al The pharmacodynamic effects of single inhaled doses of formoterol, tiotropium and their combination in patients with COPD. Pulm Pharmacol Ther 20041735–39. [DOI] [PubMed] [Google Scholar]
- 42.Cazzola M, Centanni S, Santus P.et al The functional impact of adding salmeterol and tiotropium in patients with stable COPD. Respir Med 2004981214–1221. [DOI] [PubMed] [Google Scholar]
- 43.O'Donnell D E, Fluge T, Gerken F.et al Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J 200423832–840. [DOI] [PubMed] [Google Scholar]
- 44.Hasani A, Toms N, Agnew J E.et al The effect of inhaled tiotropium bromide on lung mucociliary clearance in patients with COPD. Chest 20041251726–1734. [DOI] [PubMed] [Google Scholar]
- 45.McNicholas W T, Calverley P M, Lee A.et al Long‐acting inhaled anticholinergic therapy improves sleeping oxygen saturation in COPD. Eur Respir J 200423825–831. [DOI] [PubMed] [Google Scholar]
- 46.Cazzola M, Noschese P, Salzillo A.et al Bronchodilator response to formoterol after regular tiotropium or to tiotropium after regular formoterol in COPD patients. Respir Med 200599524–528. [DOI] [PubMed] [Google Scholar]
- 47.Baloira Villar A, Vilarino Pombo C. [Bronchodilator efficacy of combined salmeterol and tiotropium in patients with chronic obstructive pulmonary disease]. Arch Bronconeumol 200541130–134. [DOI] [PubMed] [Google Scholar]
- 48.Maltais F, Hamilton A, Marciniuk D.et al Improvements in symptom‐limited exercise performance over 8 h with once‐daily tiotropium in patients with COPD. Chest 20051281168–1178. [DOI] [PubMed] [Google Scholar]
- 49.Kim S J, Kim M S, Lee S H.et al A comparison of tiotropium 18 μg, once daily and ipratropium 40 μg, 4 times daily, in a double‐blind, double‐dummy, efficacy and safety study in adults with chronic obstructive pulmonary disease (Korean). Korean Tuberc Respir Dis 200558498–506. [Google Scholar]
- 50.van Noord J A, Aumann J L, Janssens E.et al Comparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPD. Eur Respir J 200526214–222. [DOI] [PubMed] [Google Scholar]
- 51.Casaburi R, Briggs D D, Donohue J F.et al The spirometric efficacy of once‐daily dosing with tiotropium in stable COPD: a 13‐week multicenter trial. The US Tiotropium Study Group. Chest 20001181294–1302. [DOI] [PubMed] [Google Scholar]
- 52.Donohue J F, Menjoge S, Kesten S. Tolerance to bronchodilating effects of salmeterol in COPD. Respir Med 2003971014–1020. [DOI] [PubMed] [Google Scholar]
- 53.Tashkin D, Kesten S. Long‐term treatment benefits with tiotropium in COPD patients with and without short‐term bronchodilator responses. Chest 20031231441–1449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.