Abstract
Background
Hostility and anger are risk factors for, or co‐occur with, many health problems of older adults such as cardiovascular diseases, all‐cause mortality, and asthma. Evidence that negative emotions are associated with chronic airways obstruction suggests a possible role for hostility in the maintenance and decline of pulmonary function. This study tests the hypothesis that hostility contributes to a faster rate of decline in lung function in older adults.
Methods
A prospective examination was undertaken of the effect of hostility on change in lung function over time. Data are from the VA Normative Aging Study, an ongoing cohort of older men. Hostility was measured in 1986 in 670 men who also had an average of three pulmonary function examinations obtained over an average of 8.2 years of follow up. Hostility was ascertained using the 50‐item MMPI based Cook‐Medley Hostility Scale. Pulmonary function was assessed using spirometric tests to obtain measures of forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC).
Results
Baseline pulmonary function differed between high and medium/low hostility groups (mean (SE) percent predicted FEV1 88.9 (18.5) v 95.3 (16.9) and FVC 92.5 (16.5) v 98.9 (15.9), respectively; p<0.01 for both). This overall association between higher hostility and reduced lung function remained significant after adjusting for smoking and education, although the effect size was attenuated for both FEV1 and FVC. Higher hostility was associated with a more rapid decline in lung function, and this effect was unchanged and remained significant for FEV1 in multivariate models but was attenuated for FVC. Each standard deviation increase in hostility was associated with a loss in FEV1 of approximately 9 ml/year.
Conclusions
This study is one of the first to show prospectively that hostility is associated with poorer pulmonary function and more rapid rates of decline among older men.
Keywords: psychological factors, hostility, anger, pulmonary function, risk factor
Despite evidence that emotions are associated with chronic airways obstruction, little work has been undertaken on the role of psychological factors in the growth and decline of pulmonary function.1,2 Variability in pulmonary function with aging is significant and reflects factors that influence both the development of healthy lung function and the rate of decline. Accelerated rates of pulmonary function decline are associated with increased risk for a variety of poor health outcomes, including premature mortality.3,4,5,6 Epidemiological studies have suggested that smoking, occupational exposures, familial factors (genetic or possibly prenatal influences), childhood illnesses, and air pollution play a role in both lung growth and development and pulmonary function decline. These factors, however, account for a relatively small proportion of risk, suggesting that as yet unidentified risk factors need to be further explored. Hostility and anger are considered to be risk factors for, or to co‐occur with, many health problems of older adults such as cardiovascular diseases, all‐cause mortality, and altered immune system function,7,8,9 although studies have not found uniformly positive associations with these outcomes.10 Evidence that emotions are associated with inflammatory processes11 as well as chronic airways obstruction12 suggests a possible role for psychological factors in the growth and decline of pulmonary function. However, the possible role of hostility in shaping patterns of change in pulmonary function has not been explored.
Several studies have linked short term changes in mood with transitory changes in lung function.13,14 In an examination of lung function over a longer period of time, we have shown that pessimism is related to lower levels of forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) and prospectively related to accelerated rate of lung function decline among older men.2 Some studies have identified an inverse association between negative emotions and pulmonary function in people with pre‐existing respiratory diseases such as asthma15 or chronic obstructive pulmonary disease (COPD).16 One recent study reported that hostility was consistently associated with reduced pulmonary function using a cross‐sectional design in a sample of young adults.17 However, we know of no published research that has examined the prospective association of hostility with decline in pulmonary function. The present study seeks to address this gap and tests the hypothesis that hostility contributes to faster rates of decline in lung function among older adults, controlling for covariates known to be associated with lung function and also linked to hostility or negative emotion.
Methods
The Normative Aging Study (NAS) is a longitudinal study of aging established by the Veterans Administration (VA) in 1961.18 The study cohort consists of 2280 predominantly white community dwelling men from the Greater Boston area aged 21–80 years at the time of entry. Volunteers were screened at entry according to specific health criteria,18 and those with chronic health conditions including asthma, chronic bronchitis, and chronic sinusitis were excluded. The study protocol was approved by the Human Studies Subcommittee of the Department of Veterans Affairs Medical Center and informed consent was obtained from all subjects.
Assessment of hostility
Hostility was assessed using the 50‐item MMPI based Cook‐Medley Hostility Scale (Ho)19 taken from Form AX of the MMPI.20 Items assess suspiciousness, resentment, and cynical mistrust, and respondents rated each item as either true or false. Prior research has suggested that the scale demonstrates good validity21 and reliability, with cross‐time correlations highly stable in individuals aged over 40.22 Internal consistency reliability of the scale in the present sample was 0.7. Hostility scores were analysed as a continuous variable with higher scores on the scale indicative of higher hostility levels. For some analyses we also categorised hostility score into tertiles (high, moderate, low) based on the distribution of scores in this cohort.
Form AX of the MMPI, which includes items from both the MMPI and the MMPI‐2, was administered by mail to all active cohort members (n = 1881) in 1986.20 1550 men responded (82.4% response rate), of whom 1472 provided complete and valid questionnaire data (95% of those responding). Comparison of men included in the study with those excluded (based on whether they completed the questionnaire) indicated that the non‐responders were somewhat younger and more likely to be current smokers, but did not differ on level of education or pulmonary function. Men were included in the present study if they had a pulmonary examination within 1 year of completing the MMPI and at least one follow up examination. This resulted in a study population of 670 men.
Measurement of other risk factors for pulmonary function impairment
Every 3–5 years, participants in the NAS were seen for a comprehensive examination that included a medical history and physical examination, electrocardiogram, chest radiograph, blood and urine tests, and spirometry. Before the examination participants were instructed to refrain from eating or drinking after midnight and to refrain from smoking after 20.00 hours on the previous night. Cigarette smoking status (current, former, never) was ascertained by a trained interviewer. Current smokers were defined as men who smoked ⩾1 cigarette per day. Weight and height were measured with participants wearing only socks and underpants, from which body mass index (weight/height2) is calculated. Participants also reported whether they had completed education beyond high school (yes/no).
Assessment of pulmonary function
At baseline, this study included measures of pulmonary function obtained within 1 year of the 1986 survey. Included subjects were followed for a mean (SD) of 8.22 (1.98) years. The mean number of spirometric tests was 3.5 (range 2–4). FVC manoeuvres were performed in the standing position without a noseclip using an 8 litre water‐filled spirometer (Warren E Collins Inc, Braintree, MA, USA). Acceptability of the spirometric tests was judged according to American Thoracic Society standards.23 Up to eight spirometric tests were performed until at least three acceptable tests were obtained from each subject, of which at least two were reproducible (FEV1 and FVC within 5%). Predicted values for FEV1 and FVC were calculated using regression equations relating each spirometric index to age and height among 215 asymptomatic lifetime non‐smokers in the NAS cohort.
Data analysis
Data were examined using hierarchical linear modelling (HLM; also known as random effects modelling) using repeated measures analysis in the Statistical Analysis System (PROC MIXED; SAS).24 We estimated parameters for the effect of hostility on pulmonary function over time, using continuous variables for both indices. HLM techniques are appropriate for examining both individual time paths (change in pulmonary function over time) and whether the amount of change over time varies depending on a fixed effect (level of hostility).25 Multiple observations at different times are formally viewed as nested within the individual. After variance in individual intercepts and slopes has been examined, a conditional model predicts intercept and slope terms using group as a predictor variable. In these models, individuals do not need to have the same number of observations from which to calculate change over time. Using this method, we can account for the correlation between levels of pulmonary function measured in the same individual across examinations, baseline pulmonary function levels, and also control for effects of smoking and other potential confounders.25 The covariance structure for the pulmonary function data was specified using a compound symmetry model, a structure that specifies constant variance and covariance which was found to be the best fit.
As we were primarily interested in the “between group” effects (comparing more versus less hostile people), we present data only for fixed effects. To determine whether hostility influenced the rate of decline in pulmonary function we created interaction terms for hostility and time (all variables were centred). Parameter estimates represented by these interaction terms may be interpreted as the change in pulmonary function (ml) per standard deviation change in hostility per year. Because change in pulmonary function was not normally distributed, we also ran analyses using log transformed FEV1 and FVC scores. Findings with these analyses were largely similar but significantly stronger than those based on analyses with raw FEV1 and FVC scores. However, for ease of interpretation and following previous studies using this analytical method,26,27 we report the results using raw scores. To simplify presentation in the figures, we initially divided the sample into tertiles based on the distribution of hostility scores. Examination of effects using ANOVA techniques testing differences across groups showed that trends for moderate and low hostility were similar, with pulmonary function in both groups generally significantly different from the high hostility group but not from each other. For parsimony, we combined the low and moderate groups and present results using a dichotomised hostility score (using cut points 7–20 and 20.2–37).
Results
The mean (SD) age of the sample at baseline was 62.0 (6.9) years (range 45–86). The mean (SD) hostility score was 18.5 (5.0) (range 7–37), and the distribution was somewhat skewed toward lower hostility scores. We examined the relationship between risk factors for pulmonary function impairment and hostility for the full sample at baseline. As shown in table 1, age, height, smoking status, and education did not significantly vary according to hostility level, as indicated by t tests (age, height) and χ2 analyses (smoking status, education). Although not significant, more hostile men appeared somewhat more likely to be ever smokers. We also examined the association between hostility and mean FEV1 and FVC at each examination, controlling for age and height, using analysis of covariance (table 1). While percentage predicted values of FEV1 and FVC were in the normal range at all levels of hostility, more hostile men had significantly lower levels of FEV1 and FVC than less hostile men at baseline and every other examination over the follow up period.
Table 1 Distribution of pulmonary function risk factors and pulmonary function (ml) according to level of hostility at baseline.
| Risk factor | High hostility (n = 214) | Medium/low hostility (n = 455) |
|---|---|---|
| Age (years) | 61.9 | 62.3 |
| Height (inches) | 68.7 | 68.5 |
| Current smokers (%) | 16% | 11% |
| Former smokers (%) | 57% | 51% |
| Education beyond high school (%) | 89% | 91% |
| Mean (SE) pulmonary function (ml) | ||
| FEV1 | ||
| Exam 1* | 2972.44 (37.56) | 3176.59 (25.28) |
| Exam 2* | 2873.62 (38.85) | 3047.89 (26.15) |
| Exam 3* | 2765.96 (42.64) | 2970.35 (28.28) |
| Exam 4* | 2688.35 (47.18) | 2912.56 (29.90) |
| % predicted FEV1 at baseline* | 88.9 (18.5) | 95.3 (16.9) |
| FVC | ||
| Exam 1* | 3805.77 (42.26) | 4074.28 (28.44) |
| Exam 2* | 3780.30 (44.03) | 3994.42 (29.63) |
| Exam 3* | 3644.81 (49.07) | 3911.18 (32.55) |
| Exam 4* | 3567.73 (54.73) | 3838.30 (34.69) |
| % predicted FVC at baseline* | 92.5 (16.5) | 98.9 (15.9) |
Tests of significance were conducted using χ2, t tests, or analysis of covariance, controlling for age and height (FEV1, FVC).
*Differences significant at p<0.01.
We also examined whether hostility was related to the punctuality of the medical assessment to determine whether less hostile men came in sooner for their physical examination than more hostile men. If this was true, such differences might influence any differences seen in pulmonary function. We found no relationship between punctuality of medical assessment and hostility, and therefore did not include it as a variable in subsequent analyses.
Standard control variables including age, height, education, and smoking status were adjusted for in the hierarchical linear models. The mixed regression models indicated a strong main effect for hostility (tables 2 and 3). There was an inverse association between the hostility score and pulmonary function, suggesting that more hostile men had significantly lower levels of FEV1 (b = −102.41, standard error (SE) = 20.71, p<0.001) and FVC (b = 126.14, SE = 22.98, p<0.001) when age, height, and time between assessment of hostility and each pulmonary examination (years of follow up) were adjusted for (model 1, tables 2 and 3). Parameter estimates were somewhat attenuated but remained significant after adding smoking status and education to the models (model 2, tables 2 and 3). Age, height, years of follow up, education, and smoking were all significantly related to both FEV1 and FVC in the expected directions.
Table 2 Fixed effects of hostility on FEV1 (ml) at baseline and over time.
| Effects | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| Coefficient | SE | p value | Coefficient | SE | p value | |
| Initial status (intercept) | −946.84 | 663.30 | 0.1539 | −143.88 | 654.74 | 0.8261 |
| Hostility | −102.41 | 20.71 | 0.0001 | −83.28 | 20.30 | 0.0001 |
| Hostility × time | −9.08 | 3.49 | 0.0093 | −9.36 | 3.52 | 0.008 |
| Time† | −127.89 | 3.52 | 0.0001 | −128.20 | 3.55 | 0.0001 |
| Age | −30.79 | 3.17 | 0.0001 | −37.40 | 3.15 | 0.0001 |
| Height | 84.71 | 8.55 | 0.0001 | 79.75 | 8.44 | 0.0001 |
| Current smoker‡ | 570.89 | 69.69 | 0.0001 | |||
| Former smoker¶ | −133.04 | 45.64 | 0.0037 | |||
| Education (>HS) | 106.37 | 70.87 | 0.1339 | |||
| N§ | 2386 | 2309 | ||||
†Time, time between assessment of hostility and pulmonary examination.
‡Effects are relative to never smokers (1, current smoker, 0, otherwise).
¶Effects are relative to never smokers (1, former smoker, 0, otherwise).
§These are the number of observations (multiple examinations for each individual) used in the analysis. 77 observations were dropped in analyses for model 2 because of missing information on covariates.
Table 3 Fixed effects of hostility on FVC (ml) at baseline and over time.
| Effects | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| Coefficient | SE | p value | Coefficient | SE | p value | |
| Initial status (intercept) | 2689.24 | 735.7 | 0.0003 | −2068.09 | 747.95 | 0.0059 |
| Hostility | −126.14 | 22.98 | 0.0001 | −111.14 | 23.19 | 0.0001 |
| Hostility × time | −7.98 | 4.89 | 0.10 | −7.23 | 4.95 | 0.1442 |
| Time† | 116.46 | 4.94 | 0.0001 | −115.98 | 4.98 | 0.0001 |
| Age | −33.43 | 3.52 | 0.0001 | −38.09 | 3.59 | 0.0001 |
| Height | 125.76 | 9.49 | 0.0001 | 120.64 | 9.64 | 0.0001 |
| Current smoker‡ | −408.03 | 79.64 | 0.0001 | |||
| Former smoker¶ | −98.92 | 52.14 | 0.0582 | |||
| Education (>HS) | 141.46 | 80.99 | 0.0812 | |||
| N§ | 2386 | 2309 | ||||
†Time, time between assessment of hostility and pulmonary examination.
‡Effects are relative to never smokers (1, current smoker, 0, otherwise).
¶Effects are relative to never smokers (1, former smoker, 0, otherwise).
§These are the number of observations (multiple examinations for each individual) used in the analysis. 77 observations were dropped in analyses for model 2 because of missing information on covariates.
Our primary research question concerned whether the rate of decline in pulmonary function was faster for more hostile than for less hostile men. Using an interaction term with hostility and time, we were able to test formally whether the rate of decline depended on the hostility level. In the base model, controlling for age, height, and time to examination, a significant interaction term (b = −9.08, SE = 3.49, p<0.01) suggested that more hostile individuals experienced a faster rate of decline in FEV1 than less hostile individuals, and this finding remained strong after adjustment for smoking and education level (table 2). This represents a decrease of approximately 9 ml per year in FEV1 with each standard deviation increase in hostility. The effects were similar but slightly attenuated for FVC. In the base model, the interaction term (b = −7.98, SE = 4.89, p = 0.10) suggested that hostile individuals experienced a somewhat faster rate of decline in FVC than less hostile individuals, although the effect was further attenuated after adjustment for smoking and education level (table 3).
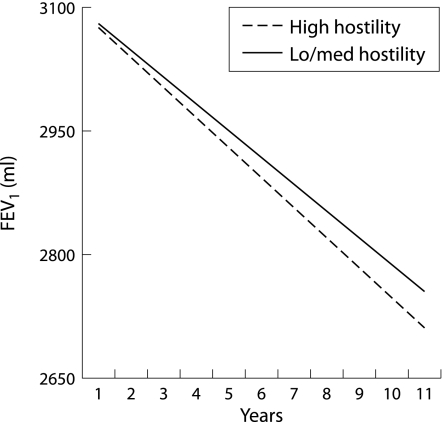
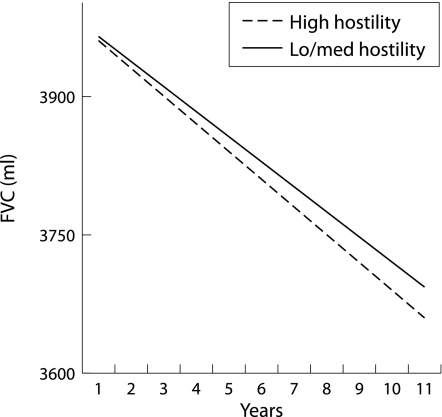
To understand the interaction terms more clearly, we used the dichotomised (low/medium versus high) hostility scores to examine rates of decline among men with high levels compared with those with moderate or lower levels of hostility (assuming equal lung function at the outset). Figure 1 shows that greater hostility was associated with a faster decline in FEV1. The findings for FVC were similar but attenuated (fig 2). These analyses suggest that more hostile people have lower levels of lung function at the outset, and also a faster rate of decline in lung function over time relative to their less hostile counterparts. For example, the decline in FEV1 in hostile individuals was approximately 37 ml/year compared with 32 ml/year in less hostile individuals. This difference in rate of decline is comparable to that found between male current smokers and never smokers in other studies, with differences of 5–7 ml/year.26 The results for FVC were similar but of a smaller magnitude.
Figure 1 Effect of hostility on forced expiratory volume in 1 second (FEV1) over time.
Figure 2 Effect of hostility on forced vital capacity (FVC) over time.
A number of potential confounders that need to be considered in the relationship between hostility and lung function and lung function decline, including body mass index (BMI), smoking dose, presence of coronary heart disease (CHD), and pessimism—a risk factor for lung function decline identified in earlier work with this sample.[2] We consider each of these potential confounders, but do not show results when the findings are not substantially changed from those reported above. Men with more hostility also had higher BMI (mean (SD) 27.27 (3.31) kg/m2) than those with low/medium hostility (26.49 (3.17) kg/m2, t (668) = −2.91, p = 0.004). However, when BMI was included as a covariate, the results for the association between hostility and lung function were only slightly attenuated; all independent effects that were seen in the original models were maintained. Similarly, we considered the possibility of residual confounding in models that controlled only for smoking status without taking account of dose. Although individuals with high hostility were somewhat more likely to smoke more (mean 24.3 pack years) than medium/low hostile individuals (mean 22.3 pack years), this difference was not significant (t (668) = −0.81, p = 0.41). Moreover, in models controlling for pack years instead of smoking status, associations between hostility and lung function were unchanged. After adjusting for effects of smoking dose on rate of decline over time for both FEV1 and FVC, an inverse association between hostility score and pulmonary function was maintained (FEV1: b = −88.56, SE = 20.31, p<0.0001; FVC: b = −113.31, SE = 22.99, p<0.0001); the rate of decline in pulmonary function was still faster for more hostile men with regard to FEV1 (b = −8.49, SE = 3.52, p = 0.02) but effects were attenuated for FVC (b = −6.33, SE = 4.95, p = 0.20). In stratified analyses, the effects of hostility on the rate of decline in lung function were similar in direction to the original findings, with some differences in the magnitude of effects. Hostility was inversely associated with FEV1 and FVC in both never and ever smokers, but in never smokers the associations were not significant. However, the effects of hostility on decline in lung function were strong in never smokers (FEV1: b = −12.95, SE = 6.28, p = 0.04; FVC: b = −22.21, SE = 8.85, p = 0.01) despite the significantly reduced sample size (n = 246); in ever smokers the effects were still clearly evident for FEV1 (b = −7.93, SE = 4.25, p = 0.06) but were highly attenuated for FVC (b = −0.89, SE = 5.94, p = 0.88). Finally, the effects of hostility on lung function were also unchanged when men with CHD diagnosed in 1986 were excluded from the analyses or when models included pessimism as a covariate.
Discussion
To our knowledge these prospective findings are the first to demonstrate an association between hostility and rate of decline in pulmonary function, an effect that was consistent for FEV1 and FVC and independent of smoking status. Higher levels of hostility were associated with both lower levels of pulmonary function at baseline and also with a faster rate of decline in lung function over time. It is interesting to note that, among more hostile men, pulmonary function was worse at every examination over a 10 year period than in less hostile men.
While it is possible that higher levels of pulmonary function lead to lower levels of hostility, several factors argue against this explanation. Firstly, the longitudinal finding of an effect of hostility on rate of decline after taking account of initial levels of pulmonary function suggests that hostility influences changes in lung function rather than vice versa. In addition, the levels of lung function were in the normal range at the outset of the study, making it less likely that poor lung function could lead to hostility. Although we measured hostility at only a single time point, the Cook‐Medley Hostility Scale was designed as a trait measure and, among older adults, levels tend to be relatively unchanging over a long span of time.22 It could be that there is a third factor leading to hostility, low pulmonary function, and faster rates of pulmonary function decline. While the current analyses were able to control for some plausible third factors such as age or socioeconomic status, others are possible.
The findings of this study are consistent with other work that has found negative cognitions, emotions, and behaviours to be associated with poorer and more rapid decline in lung function.2,17 Chronic lung problems and diseases are increasingly being viewed as inflammatory disorders.28 In the light of other work reporting an association between hostility and immune function, direct pathways linking hostility to lung function may involve influences on underlying chronic inflammatory processes. Such processes may be regulated through complicated immune phenomena in which many cells (such as neutrophils, eosinophils, and T lymphocytes) and associated cytokines play a role. For example, Miller and colleagues7 found increased natural killer cell numbers and cytotoxicity among hostile men than in less hostile men when engaged in marital conflict. Studies in patients with COPD have found a reduced CD4/CD8 ratio to be associated with lower levels of lung function and degree of airflow limitation.29
Disturbances in neuroendocrine processes are related to both hostility and pulmonary function. Some evidence has linked hostility with increased sympathetic nervous system (SNS) activity as well as alterations in levels of cortisol or dysregulation of the hypothalamic‐pituitary‐adrenal (HPA) axis. For example, one study found that highly hostile men displayed more than twice the increase in cortisol excretion during daytime hours compared with less hostile men.30 While it may seem paradoxical that activation of the HPA axis by psychological stress should be considered problematic (given that the release of cortisol has known anti‐inflammatory effects), as discussed in more detail elsewhere we have come to understand that organisms need an optimal balance of neurohormones and neurotransmitters to maintain health.1 Excessive SNS activity along with disturbances in HPA axis regulation (either hypo‐ or hyper‐responsiveness) has also been linked to poorer pulmonary function.1 In a laboratory study of acute stress reactivity and pulmonary function, individuals with and without asthma were challenged with speech and mathematical tasks. Increases in plasma epinephrine (adrenaline) and norepinephrine (noradrenaline) levels in response to the stress tasks were seen in both groups, accounting for 33–51% of the variance in concurrent pulmonary function.31
Alternatively, hostility may influence health through psychosocial pathways by its influence on social interactions and health damaging behaviors. For example, high levels of hostility often produce interpersonal conflict and hostility from others, leading to the withdrawal of social support.32 Numerous studies have linked social support to a broad array of health outcomes, with more recent work demonstrating links to relevant physiological parameters including cortisol expression.33 Moreover, hostility has been consistently linked with increased likelihood of engaging in health damaging behaviors such as smoking or sedentary behaviour34 which, in turn, may influence pulmonary function. Although physical activity is a potential confounder (or pathway) linking hostility with lung function, data on physical activity are not available for this sample so we could not examine this issue.
For more than 20 years, cigarette smoking has been recognised as the single most important risk factor for an accelerated rate of decline in pulmonary function in adult life.35 Deleterious effects of cigarette smoking on lung function decline were evident, which raises the possibility of residual confounding by smoking in these analyses. Moreover, there was some indication that smokers were more hostile. However, our analyses accounted for smoking in a variety of ways and, in almost all cases, hostility remained an independent predictor of rate of pulmonary function decline. This suggests that additional and perhaps more direct mechanisms linking hostility and pulmonary function need to be considered.
The findings of the present study are limited in that they pertain to older white men and thus cannot be generalised to women, non‐white subjects, or younger populations. Other research has suggested that women have lower levels of hostility than men, and that black ethnic groups have higher levels of hostility than white subjects.36 Research to date has suggested that hostility and lung health are similarly associated for both men and women, and for black and white subjects.17 However, further work is needed to ascertain more definitively whether the association between hostility and lung function can be generalised to other groups.
Identifying factors that predict a rapid decline in pulmonary function among older adults will increase opportunities for early intervention to protect lung health. This study provides prospective evidence that hostility is associated with a more rapid rate of decline in pulmonary function among older adult men, with effects strongest for FEV1. Hypothesised mechanisms could include both direct effects on neuroendocrine and immune function as well as effects on health related behaviors. Previous research has suggested that hostility is not immutable, as a number of clinical trials have succeeded in altering (reducing) levels of hostility using psychosocial interventions.37 Thus, the role of hostility in pulmonary health deserves a closer look.
Abbreviations
BMI - body mass index
FEV1 - forced expiratory volume in 1 second
FVC - forced vital capacity
HPA - hypothalamic‐pituitary‐adrenal
SNS - sympathetic nervous system
Footnotes
This study was partly supported by a VA Medical Research Service Merit Review Award. The VA Normative Aging Study is supported by the Cooperative Studies Program/ERIC, Department of Veterans Affairs, and is a component of the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC). This study was also supported by the J D and C T MacArthur Foundation Network on Socioeconomic Status and Health. BJ was supported in part by a training grant from the national Heart, Lung, and Blood Institute (HL07427) and faculty start‐up funds from Smith College. During preparation of the manuscript RJW was supported by K08 HL04187.
The authors have no financial associations that might pose a conflict of interest in connection with this article.
References
- 1.Wright R J, Rodriguez M, Cohen S. Review of psychosocial stress and asthma: an integrated biopsychosocial approach. Thorax 1998531066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kubzansky L D, Wright R J, Cohen S.et al Breathing easy: a prospective study of optimism and pulmonary function in the Normative Aging Study. Ann Behav Med 200224345–353. [DOI] [PubMed] [Google Scholar]
- 3.Camilli A E, Burrows B, Knudson R J.et al Longitudinal changes in forced expiratory volume in one second in adults. Effects of smoking and smoking cessation. Am Rev Respir Dis 1987135794–799. [DOI] [PubMed] [Google Scholar]
- 4.Knuiman M W, James A L, Divitini M L.et al Lung function, respiratory symptoms, and mortality: results from the Busselton Health Study. Ann Epidemiol 19999297–306. [DOI] [PubMed] [Google Scholar]
- 5.Kannel W B, Hubert H, Lew E A. Vital capacity as a predictor of cardiovascular disease: the Framingham Study. Am Heart J 1983105311–315. [DOI] [PubMed] [Google Scholar]
- 6.Cook N, Albert M. Interrelationships of peak expiratory flow rate with physical and cognitive function in the elderly: MacArthur studies of successful aging. J Gerontol 199550AM317–M323. [DOI] [PubMed] [Google Scholar]
- 7.Miller G E, Dopp J M, Myers H F.et al Psychosocial predictors of natural killer cell mobilization during marital conflict. Health Psychol 199918562–571. [DOI] [PubMed] [Google Scholar]
- 8.Everson S A, Kauhanen J, Kaplan G A.et al Hostility and increased risk of mortality and acute myocardial infarction: τhe mediating role of behavioral risk factors. Am J Epidemiol 1997146142–152. [DOI] [PubMed] [Google Scholar]
- 9.Niaura R, Todaro J F, Stroud L.et al Hostility, the metabolic syndrome, and incident coronary heart disease. Health Psychol 200221588–593. [DOI] [PubMed] [Google Scholar]
- 10.Rozanski A, Blumenthal J A, Davidson K W.et al The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am Coll Cardiol 200545637–651. [DOI] [PubMed] [Google Scholar]
- 11.Wright R J, Cohen R T, Cohen S. The impact of stress on the development and expression of atopy. Curr Opin Allergy Clin Immunol 2005523–29. [DOI] [PubMed] [Google Scholar]
- 12.Ritz T, Kullowatz A. Effects of emotion and stress on lung function in health and asthma. Curr Respir Med Rev 20051209–218. [Google Scholar]
- 13.Steptoe A, Holmes R. Mood and pulmonary function in adult asthmatics: pilot self‐monitoring study. Br J Med Psychol 19855887–94. [DOI] [PubMed] [Google Scholar]
- 14.Affleck G, Apter A, Tennen H.et al Mood states associated with transitory changes in asthma symptoms and peak expiratory flow. Psychosom Med 20006261–68. [DOI] [PubMed] [Google Scholar]
- 15.Katon W J, Richardson L, Lozano P.et al The relationship of asthma and anxiety disorders. Psychosom Med 200466349–355. [DOI] [PubMed] [Google Scholar]
- 16.Crockett A, Cranston J, Moss J.et al The impact of anxiety, depression, and living alone in chronic obstructive pulmonary disease. Qual Life Res 200211309–316. [DOI] [PubMed] [Google Scholar]
- 17.Jackson B, Kubzansky L D, Cohen S.et al Does harboring hostility hurt? Associations between hostility and pulmonary functioning in the CARDIA study. Health Psychol 2006 [DOI] [PMC free article] [PubMed]
- 18.Bell B, Rose C I, Damon A. The Normative Aging Study: an interdisciplinary and longitudinal study of health and aging. Int J Aging Human Dev 197235–17. [Google Scholar]
- 19.Cook W W, Medley D M. Proposed hostility and pharisaic‐virtue scales for the MMPI. J Appl Psychol 195438414–418. [Google Scholar]
- 20.Butcher J N, Dahlstrom W G, Graham J R.et alMMPI‐2: Minnesota Multiphasic Personality Inventory‐2. Manual for administration and scoring. Minneapolis, MN: University of Minnesota Press, 1989
- 21.Smith T W, Frohm K D. What's so unhealthy about hostility? Construct validity and psychosocial correlates of the Cook and Medley Ho scale. Health Psychol 19854503–520. [DOI] [PubMed] [Google Scholar]
- 22.Shekelle R B, Gale M, Ostfeld A M.et al Hostility, risk of coronary heart disease, and mortality. Psychosom Med 198345109–114. [DOI] [PubMed] [Google Scholar]
- 23.Ferris B G J. Epidemiology standaradization project. Am Rev Respir Dis 19781181–88. [PubMed] [Google Scholar]
- 24.SAS Institute SAS/STAT Software: Changes and Enhancements through Release 6.12. Cary, NC: SAS Institute Inc, 1997
- 25.Bijleveld C C J H, van der Kamp L J T, Mooijaart A.et alLongitudinal data analysis: design, models, and methods. London: Sage Publications, 1998
- 26.Griffith K A, Sherrill D L, Siegel E M.et al Predictors of loss of lung function in the elderly: The Cardiovascular Health Study. Am J Respir Crit Care Med 200116361–68. [DOI] [PubMed] [Google Scholar]
- 27.Alfonso H S, Fritschi L, de Klerk N H.et al Effects of asbestos and smoking on the levels and rates of change of lung function in a crocidolite exposed cohort in Western Australia. Thorax 2004591052–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rich E A. The role of immune abnormalities and inflammation in causing and perpetuating COPD. In: Cherniack NS, eds. Chronic obstructive pulmonary disease. Philadelphia: Saunders, 1991
- 29.O'Shaughnessy T C, Ansari T W, Barnes N C.et al Inflammation in bronchial biopsies of subjects' chronic bronchitis: inverse relationship of CD8+ T lymphocytes with FEV1. Am J Respir Crit Care Med 1997155852–857. [DOI] [PubMed] [Google Scholar]
- 30.Pope M K, Smith T W. Cortisol excretion in high and low cynically hostile men. Psychosom Med 199153386–392. [DOI] [PubMed] [Google Scholar]
- 31.Kang D H, Fox C. Neuroendocrine and leukocyte responses and pulmonary function to acute stressors. Ann Behav Med 200022276–285. [DOI] [PubMed] [Google Scholar]
- 32.Smith T W. Concepts and methods in the study of anger, hostility and health. In: Siegman W, Smith TW, eds. Anger, hostility and the heart. Hillsdale, NJ: Erlbaum, 199423–42.
- 33.Rosal M C, King J, Ma Y.et al Stress, social support, and cortisol: inverse associations? Behav Med 20043011–21. [DOI] [PubMed] [Google Scholar]
- 34.Siegler I C, Costa P T, Brummett B H.et al Patterns of change in hostility from college to midlife in the UNC Alumni Heart Study predict high‐risk status. Psychosom Med 200365738–745. [DOI] [PubMed] [Google Scholar]
- 35.US Department of Health and Human Services The health consequences of smoking: chronic obstructive lung disease. A Report of the Surgeon General. Washington, DC: US Government Printing Office, 1984
- 36.Siegler I C. Hostility and risk: demographic and lifestyle variables. In: Siegman AW, Smith TW, eds. Anger, hostility, and the heart. Hillsdale, NJ: Lawrence Erlbaum Associates, 1994199–214.
- 37.Friedman M, Thorensen C E, Gill J.et al Alteration of type A behavior and its effect on cardiac recurrences in postmyocardial infarction patients: summary results of the Recurrent Coronary Prevention Project. Am Heart J 1986112653–665. [DOI] [PubMed] [Google Scholar]