Abstract
Despite widespread use in pharmacokinetic, drug metabolism, and pesticide residue studies, little is known about the factors governing response during reversed-phase liquid chromatography coupled with negative-ion electrospray ionization (ESI−) mass spectrometry. We examined the effects of various mobile-phase modifiers on the ESI− response of four selective androgen receptor modulators using a postcolumn infusion system. Acetic, propionic, and butyric acid improved the ESI− responses of analytes to varying extents at low concentrations. Formic acid suppressed ionization, as did neutral salts (ammonium formate, ammonium acetate) and bases (ammonium hydroxide, triethylamine) under most conditions. Two modifiers (2,2,2-trifluoroethanol, formaldehyde) that produce anions with high gas-phase proton affinity increased ESI− responses. However, the concentrations of these modifiers required to enhance ESI− response were higher than that of acidic modifiers, which is a phenomenon likely related to their low pKa values. 2,2,2-Trifluoroethanol increased response of more hydrophobic compounds but decreased response of a more hydrophilic compound. Formaldehyde improved response of all the compounds, especially the hydrophilic compound with lower surface activity. In summary, these results suggest that an ideal ESI− modifier should provide cations that can be easily electrochemically reduced and produce anions with small molecular volume and high gas-phase proton affinity.
The inherently high selectivity and sensitivity of electrospray ionization (ESI) mass spectrometry (MS) coupled with high-performance liquid chromatography (HPLC) make it a valuable tool for the complicated matrix analyses that are required for pharmacokinetic, metabolism1,2 and pesticide residue studies.3,4 Reversed-phase (RP) HPLC using mixtures of acetonitrile/water or methanol/water as mobile phase is the most commonly employed separation method for such studies. Acids are often added to the mobile phase to facilitate ionization when using positive-ion ESI. However, less is known about the factors that govern the appropriate choice of a mobile-phase modifier when using negative-ion ESI in these mobile-phase mixtures. Since a large number of small molecules are easily ionized in negative-ion mode ESI rather than positive-ion mode ESI,4 it is important to identify ways to improve or optimize negative-ion ESI response in such systems.
Weak organic acids, such as acetic or formic acid, are often added to the solution when positive-ion ESI is performed. It is commonly accepted that the presence of the acid facilitates protonation of analytes with basic functional groups in positive-ion mode.5 Therefore, it is reasonable to assume that the addition of a base would facilitate the deprotonation of analytes in negative-ion mode. However, earlier studies of ESI MS in negative-ion mode showed that volatile bases, such as ammonium hydroxide, resulted in a poor detection limit and less stability in methanolic or aqueous solutions.5 Halogenated solvents were also evaluated in this context. For example, chloride ions produced from the reduction of chloroform at electrical contacts are thought to facilitate the production of analyte anions by proton abstraction or chloride attachment.6,7 Other studies showed that fluorinated solvents can create stable deprotonated anions and improve negative-ion ESI response. For example, hexafluoro-2-propanol and 2,2,2-trifluoroethanol were used in the analysis of oligonucleotides in the negative-ion mode.5,8–10
The “wrong-way-round” electrospray ionization concept describes the occurrence of intense [M + H]+ ions during ESI of strongly basic solutions and intense [M − H]− ions during ESI of strongly acidic solutions. This phenomenon has long been observed in protein,11 peptide,12 and amino acid13 analyses. Similar pH insensitivity also exists in small-molecule negative-ion ESI. Ferrer et al.3 used an acidic mobile phase (40% acetonitrile, 24% methanol, 35.7% water, 0.3% acetic acid) and a C18 column to separate oxanilic and sulfonic acids of acetochlor, alachlor, and metolachlor. Although the acidic mobile phase was selected to achieve successful HPLC separation, these compounds were detected with negative-ion ESI without postcolumn neutralization. Wollgast et al.14 used RP HPLC/MS with negative-ion detection to identify procyanidins in chocolate. Gradient elution with 0.2% acetic acid and acetonitrile was used. In another study to identify phenolic compounds in a cocoa sample, Sánchez-Rabaneda et al.15 changed 0.2% acetic acid to 0.1% formic acid and observed that ionic strength decreased but that the signal-to-noise ratio increased in the negative-ion mode.
A similar phenomenon was observed when negative-ion ESI was used to detect selective androgen receptor modulators (SARMs) in our laboratory. Strong ESI signals were obtained under both neutral and acidic conditions.16 These SARMs are structurally related to bicalutamide but demonstrate potent and tissue-selective androgenic and anabolic activity.17–19 These compounds are not only promising candidates for new drug development but also provide ideal tools for studies to explore the mechanisms that affect negative-ion ESI response. The present study was designed to investigate the effects of different mobile-phase modifiers on negative-ion ESI response of four SARMs. The most striking discovery is that some weak acids significantly increased the negative-ion ESI response. The factors that might affect the negative-ion ESI responses, including the acidity of bulk solution, gas-phase proton affinities, and molecular volumes of the acid modifier conjugate bases, are discussed herein.
EXPERIMENTAL SECTION
Reagents
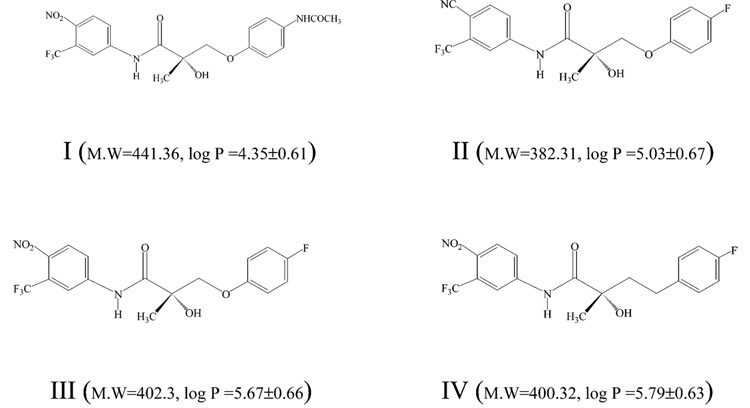
SARMs (Figure 1) were synthesized in our laboratories as previously described.19 The purities of synthesized compounds were confirmed by elemental analysis and mass spectrometry. Equimolar stock solutions of compounds I–IV were prepared by dissolving the compounds in acetonitrile/water (50:50, v/v) and then combined so that the negative-ion ESI response could be compared in the same injection, chromatographic run, and postcolumn conditions. The final concentration of each compound in the working solution was 400 nM. 2,2,2-Trifluoroethanol, triethylamine (TEA), and ammonium formate were purchased from Sigma Chemical Co. (St. Louis, MO). Acetonitrile, water, acetic acid, ammonium acetate, butyric acid, and formaldehyde were obtained from Fisher Scientific Co. (Fair Lawn, NJ). Ammonium hydroxide, formic acid, and propionic acid were purchased from J. T. Baker (Philipsburg, NJ). Stock solutions (4 M final concentration) of formic acid, acetic acid, propionic acid, butyric acid, ammonium hydroxide, TEA, formaldehyde, and 2,2,2-trifluoroethanol were prepared by adding the reagents to acetonitrile/water (50:50, v/v). Ammonium formate and ammonium acetate (4 M) were prepared in water because of their poor solubility in acetonitrile/water (50:50, v/v). Serial dilutions of the above reagents were prepared with acetonitrile/water (50:50, v/v).
Figure 1.
Chemical structures of four selective androgen receptor modulators. This series of compounds lack acidic functional groups but showed strong signals in negative-ion ESI. The presence of an NHCOCH3 functional group in I results in a compound with more hydrogen bond functionalities but a smaller hydrophobic region as compared to II–IV. The molecular weight and hydrophobicity (log P) of each compound is shown in parentheses. Log P values were calculated using ACD/Lab Log P DB by Advanced Chemistry Development, Inc., 1994–2000.
HPLC/MS Conditions
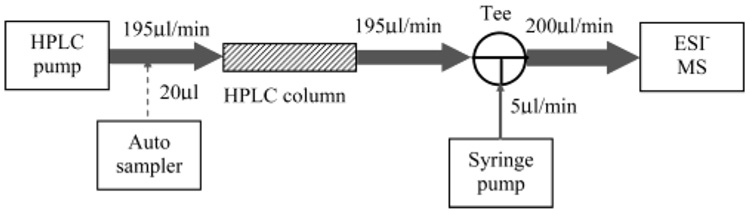
All samples were analyzed using a postcolumn infusion system (Figure 2) and a ThermoFinnigan LCQ DECA ion trap mass spectrometer (San Jose, CA) coupled with a Surveyor (ThermoQuest, San Jose, CA) HPLC system consisting of an autosampler and a quaternary pump with an online degasser. HPLC separations were performed under isocratic condition at a flow rate of 195 µL/min with acetonitrile/water (50:50, v/v) through a Vydac C8 column (208TP52 Column, C8, 300 Å, 5 µm, 2.1 mm i.d. × 250 mm, Grace Vydac, Hesperia, CA). Before entering the ESI interface, the flow was mixed with modifier, which was introduced by a syringe pump at a flow rate of 5 µL/min, by a “T” connector. Experiments were performed sequentially proceeding from lowest to highest concentration of each mobile-phase modifier. In cases where concentrations higher than 4 M were required, pure solvents were injected directly through the syringe pump. The autosampler was set at 4 °C during analysis. For the MS system, the heated capillary temperature was set at 280 °C, spray voltage was 3.5 kV, and the sheath gas and auxiliary gas flow rate were 96 and 56 mL/min, respectively. All other parameters were set to the optimized conditions for ionization and detection of III determined during preliminary experiments. Preliminary experiments (not reported) showed that the variation between optimized instrument parameters for the compounds was very small. Data acquisition was controlled by Xcalibur software. The data were collected in full-scan negative-ion mode at a range of 110-800 m/z. Chromatographic peak areas were used to compare analyte signal responses, with mass range selected as (M + 1) ± 2 (where M was the experimental m/z of [M − H]−). The injection volume was 20 µL. With the syringe pump infusing acetonitrile/water (50:50, v/v) (for blank control), the instrument was equilibrated until the ESI response of three consecutive injections became stable. The average response of the three blank runs was set as 100% response. ESI responses to different concentrations of mobile-phase modifiers were normalized to the average response of their respective blank runs. All measurements were performed in triplicate.
Figure 2.
Postcolumn infusion system. After separation on a C8 column in acetonitrile/water (50:50, v/v), four equal molar compounds were detected by ESI in negative-ion mode. The effects of different solution compositions on ESI response were tested by introducing modifier solutions via syringe pump immediately before the flow entered ESI source.
RESULTS AND DISCUSSION
Effect of Weak Acids on Negative-Ion ESI Response
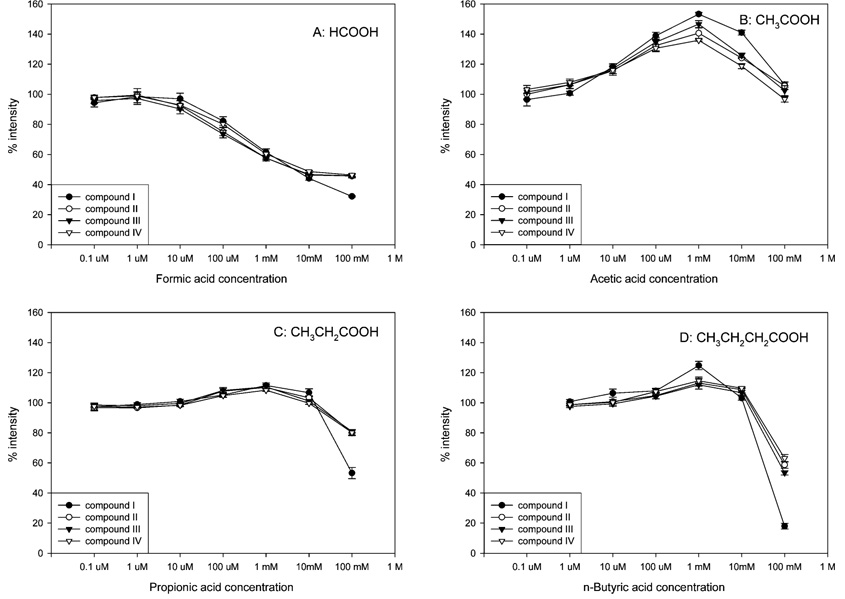
Figure 3 shows the negative-ion ESI responses of I–IV in mobile phase modified with different weak carboxylic acids, including formic acid, acetic acid, propionic acid, and n-butyric acid. When formic acid was added to the mobile phase, the negative-ion ESI responses of the four compounds decreased gradually with increasing acid concentration. This is consistent with the general idea that acidic conditions diminish negative-ion ESI response (i.e., low-pH conditions do not favor formation of the deprotonated analyte). Surprisingly, the weaker acids (i.e., acetic acid, propionic acid, and n-butyric acids) enhanced the negative-ion ESI response in concentrations ranging from 10 µM to 10 mM. The maximum ESI responses were observed at a concentration of 1 mM for these weak acids. The responses increased by about 10–50% (Figure 3B–D), with acetic acid eliciting the greatest increase in negative-ion ESI response. However, the negative-ion ESI response decreased at concentrations greater than 1 mM for all of the weakly acidic modifiers. At a concentration of 100 mM, the ESI responses of most compounds in propionic acid and n-butyric acid decreased to about 80 and 60%, respectively, of the control level. A larger decrease in the negative-ion ESI response was observed for I when 100 mM formic acid, propionic acid, or n-butyric acid was used as the modifier (Figure 3A,C,D).
Figure 3.
Effects of carboxylic acids on the negative-ion ESI responses of four SARMs. The horizontal axis represents the final concentration of modifier in the flow before entering the ESI source. The vertical axis represents the mean (±SD, N = 3) ratio of (peak area of each compound in the presence of modifier) to (the peak area of each compound in the absence of modifier), multiplied by 100%.
These results indicate that weak carboxylic acids can be used to increase the negative-ion ESI response during MS analysis of certain small molecules when used in an appropriate concentration range. The magnitude of the effect on negative-ion ESI response depends on the acidic modifier used, its concentration, and the properties of the analyte. This finding is consistent with the results previously reported by Sánchez-Rabaneda et al.15 This group showed that formic acid solutions were less effective than solutions of acetic acid when attempting to enhance the negative-ion ESI response of phenolic compounds. This suggests that this phenomenon might be commonly encountered during MS analysis of small molecules when negative-ion ESI is used. We also examined the effects of volatile bases and neutral salts on negative-ion ESI response in an attempt to better understand the underlying cause of these effects.
Free Protons from Acids Facilitate the Chemical Reduction Occurring at the Spray Tip
To accomplish successful ESI analysis, it is imperative to obtain excess charge on the droplets through an electrochemical reaction that occurs at the spray tip.20,21 In negative-ion mode, the dominant reaction is reduction. Within unmodified solutions of acetonitrile and water, protons arising from water or the analytes are presumably reduced to form hydrogen gas. The additional protons provided by an acidic modifier facilitate reduction, making it easier for the spray droplets to carry excess negative charge. This excess negative charge likely accumulates on the surface of the droplet due to electrical repulsion during negative-ion ESI, increasing the pH on the surface of the droplet and providing a local environment in which deprotonation of the analytes occurs more easily than would occur in the bulk solution.
The above hypothesis is in agreement with the reaction proposed to occur during positive-ion ESI. Gatlin and Turecek22 suggested that excess charges (e.g., protons) are confined within a thin surface layer of the droplet rather than homogeneously distributed in the bulk of the droplets, so that the local acidity of the droplet surface can be 3–4 orders of magnitude higher than that of the bulk solution during positive-ion ESI. Mansoori et al.13 collected the sprayed liquid and found that the pH of the collected sprayed solution was lower than that of the prespray solution by a few tenths of a pH unit during positive-ion ESI. Similar pH decrease was also reported by Zhou et al.23 for in situ measurement of pH using a pH-sensitive fluorophore. The electrolytic oxidation and reduction at the spray tip is one plausible explanation for the pH insensitivity observed during our negative-ion ESI analysis. ESI MS is strongly governed by the chemistry in the droplet surface layer.24 As such, the greater change in pH at the droplet surface as compared to the bulk solution may enhance negative-ion ESI response when moderate concentrations of acidic modifiers are used. However, this phenomenon may be muted at higher concentrations of the acidic modifier due to lessening of the pH gradient within the spray droplet. Therefore, analyte deprotonation would be suppressed in negative-ion mode.
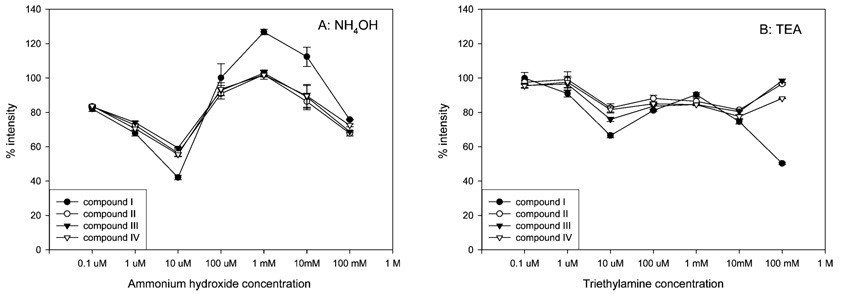
Chemical reduction at the spray tip during ESI may also provide an explanation for why the commonly used bases (e.g., ammonium hydroxide and TEA) do not facilitate deprotonation of acidic analytes. The free proton concentration in basic solutions is much lower than that in water. In addition, the cations from these bases are more difficult to reduce during electrochemical reaction. Previous studies showed that, without pneumatic assistance, negative-ion ESI analysis using basic methanolic or aqueous solutions tends to be quite unstable.5 Since stable anions are not formed to an appreciable extent, no predominant species is created to carry the negative charge at low analyte concentrations.5 We used pneumatic-assisted nebulization to stabilize ESI response during our studies. However, the negative-ion ESI response of our compounds was not enhanced when bases were added into the mobile phase.
The negative-ion ESI responses of I–IV in mobile phase modified with ammonium hydroxide and TEA are shown in Figure 4. The negative-ion ESI response of all the compounds decreased approximately 40–60% of that observed in the absence of modifier as the concentration of ammonium hydroxide approached 10 µM. Interestingly, the response returned to the control level when the concentration was increased to 1 mM and then decreased again at concentrations higher than 1 mM. TEA was unable to improve the negative-ion ESI response at any concentration tested and resulted in signal suppression in most instances (Figure 4B). As we observed in our studies using acidic modifiers (Figure 3), I showed the greatest increases and decreases in negative-ion ESI response when bases were added to the mobile phase, suggesting that other factors may also be involved in regulation of signals in negative-ion ESI. Although the basicity of ammonia and TEA might have some favorable effects on analyte deprotonation, their basicity also appears to diminish proton concentration and make it more difficult to form the excess negative charges required for ionization. These data support the idea that the presence of moderate concentrations of protons facilitate negative-ion ESI.
Figure 4.
Effect of volatile bases on negative-ion ESI responses of four SARMs. The horizontal axis represents the final concentration of modifier in the flow before entering the ESI source. The vertical axis represents the mean (±SD, N = 3) ratio of (peak area of each compound in the presence of modifier) to (the peak area of each compound in the absence of modifier), multiplied by 100%.
Considering our results with acidic and basic modifiers during negative-ion ESI, it appears that the acids provided H+ that could be easily reduced at the spray tip, while the bases suppressed ionization of I–IV via an opposing effect. This suggested that compounds that can provide cations should also be easily reduced at the spray tip and enhance negative-ion ESI.
Gas-Phase Proton Affinities of Mobile-Phase Modifier Anions Play Important Roles in Negative-Ion ESI
Cech and Enke25 previously showed that the gas-phase proton affinity of analyte is an important factor in positive-ion ESI response. We tested whether the gas-phase proton affinity of the anion of the mobile-phase modifier was important in negative-ion ESI response. The formation of excess negative charges does not guarantee high negative-ion ESI response for an analyte. Similar to positive-ion ESI,26 proton transfer resulting in a deprotonated analyte must also occur. In the present study, especially in acidic conditions, it was obvious that the negative charges were mainly carried by the conjugate base anions (which are the deprotonated acid modifiers) on the droplets after they were formed during electrospray. If proton transfer had not occurred, then either no change or even a suppression of the analyte response would have been observed in our studies and different acids would have resulted in similar negative-ion ESI response for each analyte. However, as shown in Figure 3, different acids had quite different effects on the negative-ion ESI responses of these compounds, indicating that the properties of the acid played an important role in analyte deprotonation.
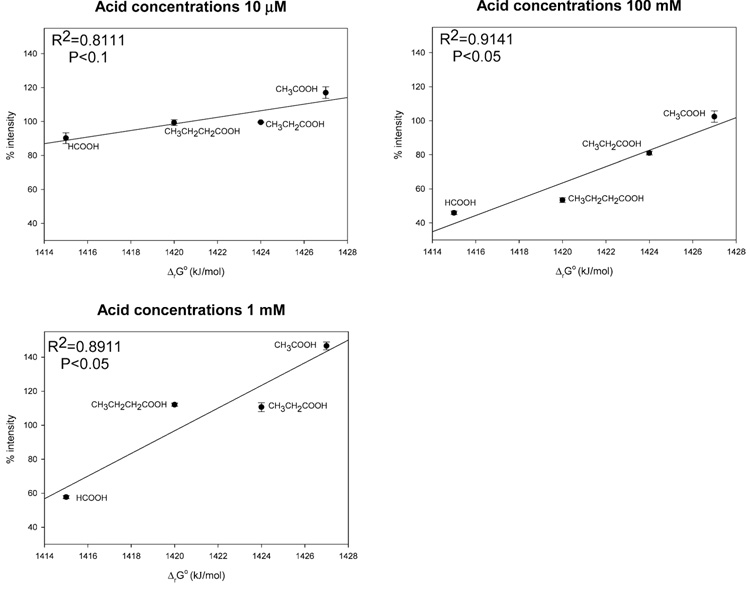
Table 1 lists the Gibbs free energies of formation (i.e., the gas-phase proton affinity) and the pKa (−log acid dissociation constant) for the modifiers that we used in these studies. The solution-phase pKa of these modifiers do not vary in direct proportion to their gas-phase proton affinity. Acetic acid has the highest gas-phase proton affinity, whereas formic acid has the lowest gas-phase proton affinity among the four acidic modifiers that we tested. To simplify, III was selected to demonstrate the relationship between the Gibbs free energy of acid formation in the gas phase and the negative-ion ESI response. Figure 5 shows the relationship between the gas-phase proton affinities of the acidic modifiers and the negative-ion ESI response of III at three different acid concentrations (10 µM, 1 mM, 100 mM). The negative-ion ESI response of III increased with the gas-phase proton affinities of the acids at all three concentrations. Thus, it appears that the protons from acids simply provide cations for electrochemical reduction. The acid modifier conjugate bases carry the excess negative charges on the surface of the droplets when the analytes have lesser acidity and contribute significantly to the deprotonation of analytes entering the gas phase. Therefore, even though the ionization process in negative-ion ESI seemed to be pH insensitive, the composition of the solution and the properties of the modifiers elicit important effects on the ESI response.
Table 1.
pKa Values of Some Reagents Used in the Present Study and Their Gas-Phase Gibbs Free Energies of Formation
| compounds | pKa27 | reaction | ΔrG° (kJ/mol) |
|---|---|---|---|
| formic acid | 3.75 | HCOO− + H+ = HCOOH | 1415.0 ± 8.4 28 |
| acetic acid | 4.746 | CH3COO− + H+ = CH3COOH | 1427.0 ± 8.4 29 |
| propionic acid | 4.87 | CH3CH2COO− + H+ = CH3CH2COOH | 1424.0 ± 8.4 30 |
| n-butyric acid | 4.83 | CH3CH2CH2COO− + H+ = CH3CH2CH2COOH | 1420.0 ± 8.4 28 |
| 2,2,2-trifluoroethanol | 12.37 | CF3CH2O− + H+ = CF3CH2OH | 1482.0 ± 8.4 30 |
| formaldehyde | 13.27 | CHO− + H+ = HCOH | 1618.0 ± 1.3 31 |
| water | 13.995 | HO− + H+ = H2O | 1605.4 ± 1.3 32 |
Figure 5.
Relationship between Gibbs free energies of formation of acids from anions and protons in gas phase and negative-ion ESI responses of III at three different acid concentrations. The relationships suggest that the gas-phase proton affinities of modifier anions are important to negative-ion ESI response.
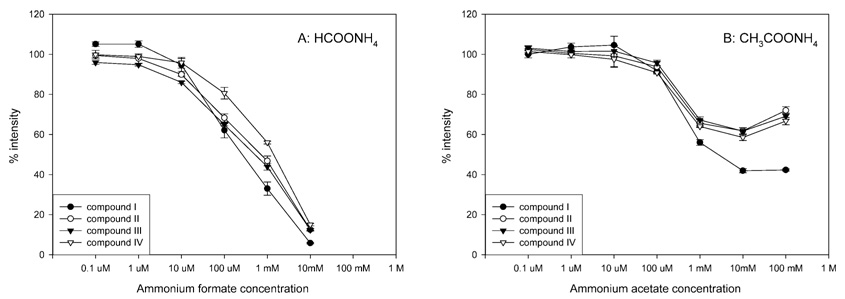
To test this hypothesis, the effects of neutral salts on negative-ion ESI response were also examined. Figure 6 shows the ESI response of I–IV when different concentrations of ammonium formate and ammonium acetate were added to the mobile phase. These neutral salts cannot provide cations (e.g., protons) to facilitate chemical reduction. Further, they do not provide a basic environment in which analyte deprotonation can occur. As predicted, no favorable effects on negative-ion ESI response were observed at any condition. The deleterious effects of neutral salts were also predicted by Enke’s equilibrium partitioning model.20 At a concentration of 10 mM, the suppressive effect of ammonium formate was so strong that the negative-ion ESI response of the four compounds decreased to less than 15% of that observed in the absence of modifier. Ammonium acetate showed milder suppressive effects on ESI response. Despite the decrease in ESI response, these data corroborate the important role of anions in negative-ion ESI. Observed differences between ESI response in the presence of the acetate and formate groups suggested that other factors might also play important roles in negative-ion ESI. Upon entering the gas phase, the acetic anion appeared to cause less suppression of analyte deprotonation as compared to the formate anion resulting in milder effects when using ammonium acetate.
Figure 6.
Effect of neutral salts on the negative-ion ESI responses of four SARMs. The horizontal axis represents the final concentration of modifier in the flow before entering the ESI source. The vertical axis represents the mean (±SD, N = 3) ratio of (peak area of each compound in the presence of modifier) to (the peak area of each compound in the absence of modifier), multiplied by 100%.
Molecular Volume of the Modifier Is Also an Important Factor Affecting ESI Response
Figure 3 showed that high concentrations of acetic acid, propionic acid, and butyric acid (10 mM, 100 mM) decreased ESI response. One might expect that the low pH caused by high concentrations of acid might suppress negative-ion ESI response of the analyte. However, one would not expect to observe different effects with different acids as we did in our studies. Acetic acid did not suppress negative-ion ESI response at the highest concentration. However, weaker acids (i.e., propionic and n-butyric acid) suppressed ionization to a measurable extent, suggesting that other factors were operative. The suppressive effect appeared to be more significant as the size of the acidic group increased.
To better understand the different effects of different acids on the analytes, the interaction between the modifiers and the analytes was considered. Molecules with nonpolar regions and ions prefer the air interface as opposed to the aqueous bulk solution inside the droplets and therefore preferentially accumulate at the droplet surface. At the same concentration, molecules or ions with larger nonpolar regions thus demonstrate higher surface affinity and are expected to occupy more of the droplet surface space. If an analyte cannot gain sufficient access to the surface, it cannot be easily charged and detected by ESI.33 In the present case, the excess charges are carried by the anion formed during electrochemical reduction. As described above, these charges tend to distribute on the surface of the droplets due to electrical repulsion. Anions with larger molecular volume might be expected to occupy a greater fraction of the droplet surface than those with smaller volumes and thus more readily compete with the analytes for the droplet surface space and have greater suppressive effects on ESI response. We determined the molecular volumes of the modifier anions used in our study and present these values in Table 2.
Table 2.
Log P Values of Some Reagents and Their Anion Volumes
| compounds | log Pa | anion | volume (ų)b |
|---|---|---|---|
| formic acid | −0.54 ± 0.19 | HCOO− | 37.74 |
| acetic acid | −0.29 ± 0.18 | CH3COO− | 54.97 |
| propionic acid | 0.25 ± 0.18 | CH3CH2COO− | 73.85 |
| n-butyric acid | 0.78 ± 0.18 | CH3CH2CH2COO− | 92.78 |
| 2,2,2-trifluoroethanol | 0.31 ± 0.55 | CF3CH2O− | 68.44 |
| formaldehyde | 0.35 ± 0.19 | CHO− | 30.58 |
| water | −1.38 ± 0.21 | HO− | 16.71 |
Log P values were calculated using ACD/Lab Log P DB by Advanced Chemistry Development, Inc., 1994–2000.
Molecular volumes were calculated from molecular (Connolly) surfaces generated using the MOLCAD module of Sybyl 6.8 on a Silicon Graphics O2 workstation. Molecular structures were energy minimized using the Tripos force field with a termination gradient of 0.05 kcal/(mol·Å) prior to surface generation.
Surface competition between the modifiers and the analytes is supported by the response of different analytes to the presence of the same mobile-phase modifier. Compared to the other three compounds, the negative-ion ESI response of I was suppressed to a greater extent when high concentrations of acid (100 mM) were used. The hydrophobicity (i.e., log P, log octanol/water partition coefficient) of I–IV are summarized in Figure 1. The p-acetamido functional group in I adds hydrogen-bonding ability, enhances aqueous solubility, and decreases hydrophobicity of this analogue as compared to II–IV, which contain a p-fluoro substituent at this position. Due to its lesser hydrophobicity, I showed shorter retention time during reversed-phase HPLC. The retention times of I–IV were 5.2, 8.7, 10.0, and 12.3 min, respectively. When only acetonitrile/water (50:50, v/v) was used in the mobile phase, the negative-ion ESI response generally increased with the retention time. This agrees with previous studies which showed that hydrophobic analytes with higher affinity for the surface of ESI droplets demonstrate higher ESI response.25,33–37 The greater hydrophilic character and lower surface activity of I, thus, made it more vulnerable to the suppressive effects of anions with larger molecular volumes. Previous positive-ion mode ESI studies also showed that the ESI response of less surface-active analytes tends to be suppressed to a greater extent than that of more surface-active analytes.25,38 Highly hydrophilic compounds, such as oligosaccharides, are not particularly well suited for ESI analysis. However, significant improvement in response can be accomplished by modifying oligosaccharides with hydrophobic species prior to ESI analysis.39,40
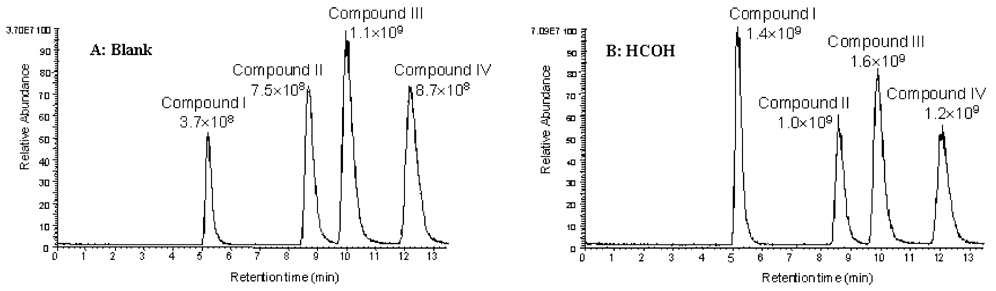
The mobile-phase modifiers’ differential effects on the negative-ion ESI response of these compounds were also observed when neutral salts (Figure 6) and bases (Figure 4) were used. Compound I always suffered worse signal suppression than the other three compounds when high concentrations of modifiers were added to the solutions. An exception is when ammonium hydroxide was used as a modifier. Only I exhibited enhanced ESI response in the presence of 100 µM-10 mM concentrations of this additive. However, it is important to note that its absolute ESI response was still the lowest among the four compounds under these conditions. This indicates that the ESI response of I was suppressed to the greatest extent in simple solution of acetonitrile and water, an observation easily confirmed by visual inspection of the chromatograms obtained in the absence of any mobile-phase modifiers (Figure 7A). Importantly, this suggests that the addition of modifiers that produce anions with smaller molecular volumes might be used to enhance negative-ion ESI response of hydrophilic analytes.
Figure 7.
Mass chromatograms of equimolar SARMs in different solution compositions. (A) Blank, unmodified acetonitrile/water (50:50, v/v) was used. (B) HCOH, Formaldehyde (100 mM) was added to the final solution just before entering the ESI source. This condition has a favorable effect for all four compounds, especially I. The compound names and their peak areas are labeled near their corresponding peaks.
Ideal Mobile-Phase Modifiers and the Effect of Weak Acid-like Compounds on Negative-Ion ESI
Based on the results above, we postulated that an ideal negative-ion ESI mobile-phase modifier for a mixture of acetonitrile and water would do the following: (1) provide cations in solution that can be electrochemically reduced and promote the formation of droplets with excess negative charge; (2) produce anions that have high gas-phase proton affinity to facilitate the deprotonation of analytes; and (3) demonstrate small anion volumes to avoid competition for surface space between the modifier and analytes.
2,2,2-Trifluoroethanol and formaldehyde were used to test this theory. 2,2,2-Trifluoroethanol is a very weak acid (pKa 12.37) that does not affect pH even at high concentrations. However, the ability of this additive to produce trifluoroethoxide and hydrogen as a result of the inherent electrochemical reduction that occurs during ESI5 suggests that it can facilitate the electrochemical reaction. The ΔrG° value of 2,2,2-trifluoroethanol is 1482 ± 8.4 kJ/mol,30 which suggests high gas-phase affinity of CF3CH2O−. However, its anion volume is higher than that of acetic anion (Table 2), suggesting that it may suppress ESI response for less surface-active analytes. Cech and Enke suggested that this compound can be used to increase signal-to-noise ratio during negative-ion ESI analysis of oligonucleotides.5 However, detailed studies to explore the mechanism of these observations were never reported. We also examined the effect of formaldehyde on the negative-ion ESI response of these four compounds. The pKa of formaldehyde is 13.27, only slightly lower than that of water (13.995). Its ΔrG° value is 1618 ± 1.3 kJ/mol,31 suggesting very strong gas-phase proton affinity, and it has a very small anion volume (30.58 ų) (Table 2), suggesting that it will not appreciably interfere with ESI due to surface-active effects.
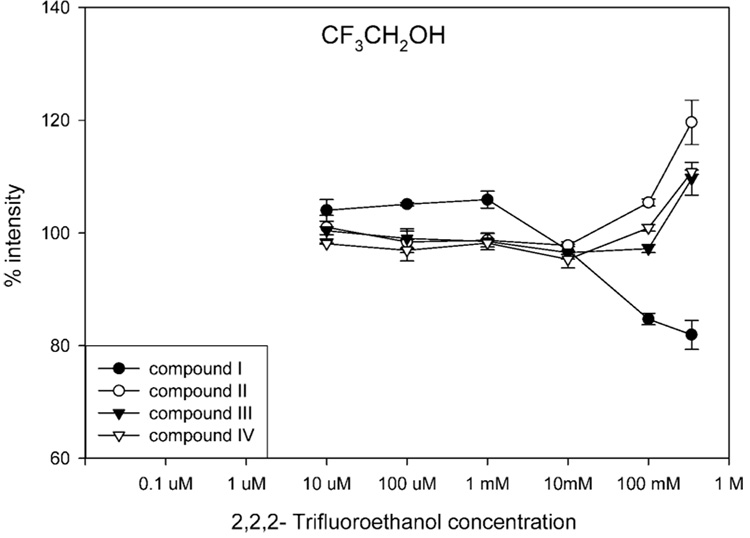
Figure 8 shows the effects of 2,2,2-trifluoroethanol on negative-ion ESI of the four SARMs. The highest concentration of 2,2,2-trifluoroethanol used was 2.5% (i.e., 346 mM). The lowest effective concentration was ~100 mM, which is much higher than that of the carboxylic acids (~10 µM) presented in Figure 3. This result supports the idea that free protons in solution can facilitate the negative-ion ESI response. Since 2,2,2-trifluoroethanol has a lower pKa than any of the carboxylic acids (see Table 1), a higher concentration of 2,2,2-trifluoroethanol was needed to provide sufficient protons to facilitate electrochemical reduction. This result is consistent with the solution composition suggested by Cech and Enke,5 who used 10% 2,2,2-trifluoroethanol in methanol for negative-ion ESI analysis of oligonucleotides. As expected, 2,2,2-trifluoroethanol showed more favorable effects on the analytes with high surface activities and suppressive effects on the more hydrophilic compound (i.e., I) with lesser surface activity.
Figure 8.
Effects of 2,2,2-trifluoroethanol on negative-ion ESI response of four SARMs. The horizontal axis represents the final concentration of 2,2,2-trifluoroethanol in the flow before entering the ESI source. The vertical axis represents the mean (±SD, N = 3) ratio of (peak area of each compound in the presence of modifier) to (the peak area of each compound in the absence of modifier), multiplied by 100%. Higher concentrations (100 mM) of 2,2,2-trifluoroethanol were required to enhance negative-ion ESI response as compared to carboxylic acids (10 µM) due to its higher pKa (12.37). 2,2,2-Trifluoroethanol enhanced ESI response for more hydrophobic analytes (i.e., II–IV) and suppressed ESI response for the less surface active I.
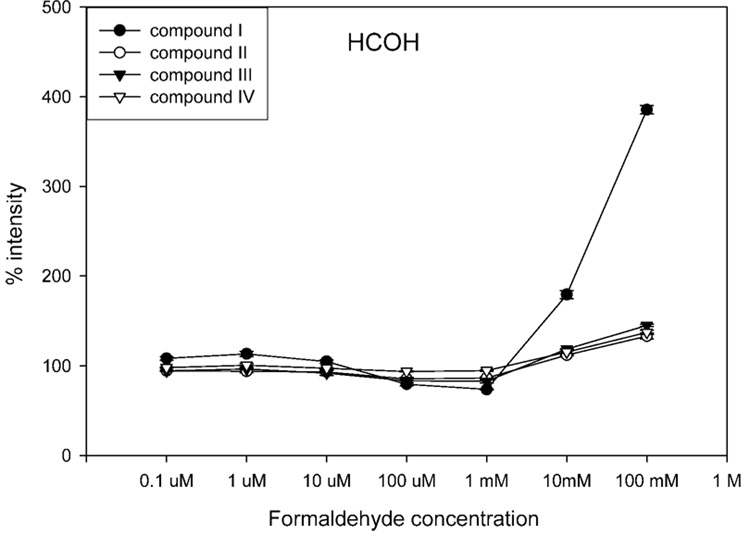
Figure 9 shows the effects of formaldehyde on negative-ion ESI of these compounds. Similarly, formaldehyde has a high pKa value (13.27) and required higher concentrations (10 mM) to enhance ESI response. Unlike 2,2,2-trifluoroethanol, it has a smaller anion volume and showed no suppressive effects on I. On the contrary, the signal of I increased by 385% when 100 mM formaldehyde was added. The enhancement of negative-ion ESI response for the more surface-active compounds (i.e., II–IV) increased 133–145%, similar to the increases (135–146%) observed when 1 mM acetic acid was used. However, it is important to reiterate that the absolute ESI response for I was less than that observed for some of the other compounds (see Figure 7B). It is generally accepted that the longer retention times during reversed-phase HPLC are associated with greater ESI response.37 Formaldehyde was able to overcome these suppressive effects to a greater extent for I, returning its ESI response to levels comparable to that of later eluting analytes and potentially corroborating the importance of anion volume of the modifier to negative-ion ESI response. One might think that the log P value would be a good predictor of the ability of a modifier to compete for the droplet surface. However, our data with formaldehyde reveal that anion volume is a more important factor influencing the ESI response. Formaldehyde has about the same log P value (0.35) as trifluoroethanol but has a much smaller anion volume and actually improved ESI response for all of the analytes. In fact, this general phenomenon was also observed upon examination of the ESI response of these analytes in the presence of different carboxylic acids (i.e., acids with smaller anion volumes were more favorable for negative-ion ESI response). This indicates that the properties of the anion (e.g., anion volume) are more important than the physicochemical properties (e.g., log P) of the neutral modifier from which they arose.
Figure 9.
Effects of formaldehyde on negative-ion ESI response of four SARMs. The horizontal axis represents the final concentration of formaldehyde in the flow before entering the ESI source. The vertical axis represents the mean (±SD, N = 3) ratio of (peak area of each compound in the presence of modifier) to (the peak area of each compound in the absence of modifier), multiplied by 100%. A higher concentration of formaldehyde (10 mM) was required to enhance negative-ion ESI response as compared to carboxylic acids (10 µM) due to its higher pKa (13.27). The small anion volume (30.58 ų) and high gas-phase proton affinity (ΔrG°, 1618 kJ/mol) of its anion resulted in significant enhancement of negative-ion ESI response for all of the compounds, particularly the less surface-active I.
CONCLUSION
In this study, we found that weak acids significantly increased the negative-ion ESI responses of four model compounds. This phenomenon could be explained by the high gas-phase proton affinity of their anions, with the free protons in acidic solution acting only as counterions that can be easily reduced during negative-ion ESI. The molecular volume of the anion was also identified as an important factor that may affect ESI response. The negative-ion ESI response of hydrophobic analytes (e.g., II–IV) was less affected by the presence of mobile-phase modifiers that produced anions with larger molecular volumes, owing to their propensity to accumulate on the droplet surface. However, mobile-phase modifiers with large anion volumes suppressed ESI response of a more hydrophilic analyte (i.e., I), likely by preventing its access to the droplet surface. Mobile-phase modifiers with small anion volume showed lesser effects in this respect. Studies using 2,2,2-trifluoroethanol and formaldehyde confirmed the importance of these factors. In summary, our studies indicate that negative-ion ESI response of analytes in reversed-phase mobile phases is a dynamic interplay between the properties of the analyte and mobile-phase modifier. The studies reported herein provide evidence to support the idea that an ideal negative-ion ESI modifier should (1) provide cations that can be easily reduced during electrochemical reaction and (2) produce anions with high gas-phase proton affinity and (3) small molecular volumes. Acetic acid appears to meet most of these requirements due to the high gas-phase proton affinity of its anion, its small anion volume, and its high acidity. As most analytes that are suitable for negative-ion ESI contain acidic groups, acetic acid would likely prolong their retention time during reversed-phase HPLC and thus improve analyte separation and ESI response. Small modifiers such as formaldehyde may be well suited for applications involving more hydrophilic and less surface-active analytes.
ACKNOWLEDGMENT
We thank Michael Mohler for discussions of the manuscript. This research was supported in part by grants from the National Cancer Institute (R29 CA68096) and the National Institute of Diabetes, Digestive, and Kidney Diseases (R01 DK59800).
References
- 1.Yin D, Xu H, He Y, Kirkovsky LI, Miller DD, Dalton JT. J. Pharmacol. Exp. Ther. 2003;304:1323–1333. doi: 10.1124/jpet.102.040832. [DOI] [PubMed] [Google Scholar]
- 2.Kajbaf M, Barnaby RJ, Bottacini M, Bertolotti L, Ismail IM, Cholerton TJ, Pellegatti M. Xenobiotica. 2003;33:415–428. doi: 10.1080/0049825031000072469. [DOI] [PubMed] [Google Scholar]
- 3.Ferrer I, Thurman EM, Barceló D. Anal. Chem. 1997;69:4547–4553. doi: 10.1021/ac9704671. [DOI] [PubMed] [Google Scholar]
- 4.Thurman EM, Ferrer I, Barceló D. Anal. Chem. 2001;73:5441–5449. doi: 10.1021/ac010506f. [DOI] [PubMed] [Google Scholar]
- 5.Cech NB, Enke CG. Mass Spectrom. Rev. 2001;20:362–387. doi: 10.1002/mas.10008. [DOI] [PubMed] [Google Scholar]
- 6.Cole RB, Zhu J. Rapid Commun. Mass Spectrom. 1999;13:607–611. [Google Scholar]
- 7.Zhu J, Cole RB. J. Am. Soc. Mass Spectrom. 2000;11:932–941. doi: 10.1016/s1044-0305(00)00164-1. [DOI] [PubMed] [Google Scholar]
- 8.Apffel A, Chakel JA, Fischer S, Lichtenwalter K, Hancock WS. Anal. Chem. 1997;69:1320–1325. doi: 10.1021/ac960916h. [DOI] [PubMed] [Google Scholar]
- 9.Griffey RH, Greig MJ, Gaus HJ, Liu K, Monteith D, Winniman M, Cummins LL. J. Mass Spectrom. 1997;32:305–313. doi: 10.1002/(SICI)1096-9888(199703)32:3<305::AID-JMS482>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 10.Huber CG, Krajete A. J. Chromatogr., A. 2000;870:413–424. doi: 10.1016/s0021-9673(99)00953-x. [DOI] [PubMed] [Google Scholar]
- 11.Kelly MA, Vestling MM, Fenselau CC, Smith PB. Org. Mass Spectrom. 1992;27:1143–1147. [Google Scholar]
- 12.Wang G, Cole RB. Org. Mass Spectrom. 1994;24:419–427. [Google Scholar]
- 13.Mansoori BA, Volmer DA, Boyd RK. Rapid Commun. Mass Spectrom. 1997;11:1120–1130. [Google Scholar]
- 14.Wollgast J, Pallaroni L, Agazzi ME, Anklam E. J. Chromatogr., A. 2001;926:211–220. doi: 10.1016/s0021-9673(01)00994-3. [DOI] [PubMed] [Google Scholar]
- 15.Sánchez-Rabaneda F, Jáuregui O, Casals I, Andrés-Lacueva C, Izquierdo-Pulido M, Lamuela-Raventós RM. J. Mass Spectrom. 2003;38:35–42. doi: 10.1002/jms.395. [DOI] [PubMed] [Google Scholar]
- 16.Wu D, Miller DD, Dalton JT. 51th ASMS Conference on Mass Spectrometry and Allied Topics. Canada: Montreal; 2003. [Google Scholar]
- 17.Dalton JT, Mukherjee A, Zhu Z, Kirkovsky L, Miller DD. Biochem. Biophys. Res. Commun. 1998;244:1–4. doi: 10.1006/bbrc.1998.8209. [DOI] [PubMed] [Google Scholar]
- 18.Yin D, He Y, Perera MA, Hong SS, Marhefka C, Stourman N, Kirkovsky L, Miller DD, Dalton JT. Mol. Pharmacol. 2003;63:211–223. doi: 10.1124/mol.63.1.211. [DOI] [PubMed] [Google Scholar]
- 19.He Y, Yin D, Perera M, Kirkovsky L, Stourman N, Li W, Dalton JT, Miller DD. Eur. J. Med. Chem. 2002;37:619–634. doi: 10.1016/s0223-5234(02)01335-1. [DOI] [PubMed] [Google Scholar]
- 20.Enke CG. Anal. Chem. 1997;69:4885–4893. doi: 10.1021/ac970095w. [DOI] [PubMed] [Google Scholar]
- 21.VanBerkel GJ, Zhou FM, Aronson JT. Int. J. Mass Spectrom. Ion Processes. 1997;162:55–67. [Google Scholar]
- 22.Gatlin CL, Turecek F. Anal. Chem. 1994;66:712–718. [Google Scholar]
- 23.Zhou SL, Edwards AG, Cook KD, Van Berkel GJ. Anal. Chem. 1999;71:769–776. [Google Scholar]
- 24.Zhou SL, Cook KD. J. Am. Soc. Mass Spectrom. 2000;11:961–966. doi: 10.1016/S1044-0305(00)00174-4. [DOI] [PubMed] [Google Scholar]
- 25.Cech NB, Enke CG. Anal. Chem. 2000;72:2717–2723. doi: 10.1021/ac9914869. [DOI] [PubMed] [Google Scholar]
- 26.Mirza UA, Chalt BT. Anal. Chem. 1994;66:2898–2904. doi: 10.1021/ac00090a017. [DOI] [PubMed] [Google Scholar]
- 27.Lide DR, editor. CRC Handbook of Chemistry and Physics. Boca Raton, FL: CRC Press; 2001–2002. [Google Scholar]
- 28.Caldwell G, Renneboog R, Kebarle P. Can. J. Chem. 1989;67:611–618. [Google Scholar]
- 29.Taft RW, Topsom RD. Prog. Phys. Org. Chem. 1987;16:1. [Google Scholar]
- 30.Cumming JB, Kebarle P. Can. J. Chem. 1978;56:1. [Google Scholar]
- 31.Murray KK, Miller TM, Leopold DG, Lineberger WC. J. Chem. Phys. 1986;84:2520–2525. [Google Scholar]
- 32.Smith JR, Kim JB, Lineberger WC. Phys. Rev. A. 1997;55:2036–2043. [Google Scholar]
- 33.Iribarne JV, Dziedzic PJ, Thomson BA. Int. J. Mass Spectrom. Ion Phys. 1983;50:331–347. [Google Scholar]
- 34.Fenn JB. J. Am. Soc. Mass Spectrom. 1993;4:524–535. doi: 10.1016/1044-0305(93)85014-O. [DOI] [PubMed] [Google Scholar]
- 35.Tang K, Smith RD. J. Am. Soc. Mass Spectrom. 2001;12:343–347. doi: 10.1016/S1044-0305(01)00222-7. [DOI] [PubMed] [Google Scholar]
- 36.Zhou SL, Cook KD. J. Am. Soc. Mass Spectrom. 2001;12:206–214. doi: 10.1016/S1044-0305(00)00213-0. [DOI] [PubMed] [Google Scholar]
- 37.Cech NB, Krone JR, Enke CG. Anal. Chem. 2001;73:208–213. doi: 10.1021/ac0006019. [DOI] [PubMed] [Google Scholar]
- 38.Bonfiglio R, King RC, Olah TV, Merkle K. Rapid Commun. Mass Spectrom. 1999;13:1175–1185. doi: 10.1002/(SICI)1097-0231(19990630)13:12<1175::AID-RCM639>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 39.Okamoto M, Takahshi KI, Doi T. Rapid Commun. Mass Spectrom. 1995;9:641–643. doi: 10.1002/rcm.1290090804. [DOI] [PubMed] [Google Scholar]
- 40.Yoshino K, Takao T, Murata H, Shimonishi Y. Anal. Chem. 1995;67:4028–4031. doi: 10.1021/ac00117a034. [DOI] [PubMed] [Google Scholar]