Abstract
In insects, 3,4-dihydroxyphenylalanine (DOPA) is required for tanning of newly formed cuticle and the production of melanin during some types of immune responses. DOPA is produced by the hydroxylation of tyrosine, and this reaction can be catalyzed by two types of enzymes: tyrosine hydroxylase (TH) and phenoloxidase (PO). TH is required for cuticle tanning in Drosophila melanogaster and for cuticle pigmentation in other insect species, but additional functions of TH have been uncertain. In contrast, an immune function for PO has been well documented. The goal of this study was to characterize TH from Manduca sexta with a focus on its possible contribution to cuticle tanning and immune-associated melanization. We cloned a full length TH cDNA, purified recombinant TH, and confirmed that MsTH and MsPO have tyrosine hydroxylating activity. To determine possible functions, we analyzed TH expression profiles. TH mRNA and protein were present in eggs at the stage when the pharate larval cuticle begins to tan and also in the integument of molting larvae. The amount of TH in the integument was correlated with the degree of cuticle tanning. Unlike PO, which was found to be constitutively expressed by hemocytes and was present in plasma, TH was upregulated in hemocytes and the fat body in response to an immune challenge and remained intracellular. These data suggest that TH is required for cuticle tanning and immunity in M. sexta. Based on the collective information from many studies, we propose a model in which TH is a major producer of the DOPA required for both cuticle tanning and immune-associated melanization.
Keywords: phenoloxidase, tyrosine hydroxylase, hydroxylation, melanin, insect immunity, cuticle tanning, sclerotization, tyrosine, DOPA
1. Introduction
Catechols derived from tyrosine are required for many physiological processes in insects including cuticle tanning and pigmentation, wound healing, and melanization of microbes and parasites during immune responses. The first step in generating these products is the hydroxylation of tyrosine (a monophenol) to produce 3,4-dihydroxyphenylalanine (DOPA, an o-diphenol). DOPA and its derivatives such as dopamine are oxidized to their corresponding quinones, which undergo subsequent reactions leading to tanning or melanization. Two types of enzymes are thought to catalyze the hydroxylation of tyrosine in insects, tyrosine hydroxylase (TH) and phenoloxidase (PO); however, it is unknown whether TH and PO function in different physiological processes or whether they function together in some pathways.
THs are highly conserved enzymes found in both vertebrates and invertebrates. They are pterin-dependent monooxygenases (E.C. 1.14.16.2) that contain ferrous iron at the active site (Ulrich and Hofrichter, 2007). THs are cytoplasmic enzymes. All but a few of the studies of insect THs have focused on two TH isoforms expressed in Drosophila melanogaster. The isoforms are encoded by a single gene and are generated by alternative splicing of RNA (Birman et al., 1994). The longer, epidermal form contains a highly acidic, 71 amino acid region that is encoded by two alternate exons. The shorter form lacking this region is expressed in neural cells. Studies of TH mutants have demonstrated that TH is required for cuticle tanning and neural function (Budnik and White, 1987; Neckameyer and White, 1993; True et al., 1999; Friggi-Grelin et al., 2003). Recent studies have linked TH to larval pigmentation patterns in Papilio xuthus and Pseudolatia separatata by demonstrating expression of TH in the epithelial cells underlying darkly pigmented cuticle (Futahashi and Fujiwara, 2005; Ninomiya and Hayakawa, 2007). Two studies suggest that TH may have an immune function: a microarray analysis of D. melanogaster genes indicated that TH is slightly upregulated in adults after septic injury (De Gregorio et al., 2001), and TH cDNA was detected in a pool of immune induced cDNAs from the hemocytes of Galleria mellonella (Seitz et al., 2003).
Insect POs are considered to be a tyrosinase type of enzyme (E.C. 1.14.18.1). Tyrosinases have the ability to hydroxylate monophenols such as tyrosine and to oxidize o-diphenols such as DOPA (Korner and Pawelek, 1982). The hydroxylation step has complex kinetics, including a variable lag phase at the start of the reaction (Pomerantz, 1966; Molina et al., 2007). The oxidation reaction is much faster than the hydroxylation reaction and proceeds without a lag phase (Rodriguez-Lopez, 1992). Insect POs are not orthologous to the well characterized mammalian and fungal tyrosinases (van Holde et al., 2001), but they have similar activities (reviewed in Ashida and Brey, 1997). All of the insect POs that have been tested exhibit o-diphenoloxidase activity, but only a subset were observed to hydroxylate monophenols. The best demonstration of hydroxylating activity of an insect PO is a study by Chase et al. (2000) that analyzed the activity of PO from the hemolymph of Sarcophaga bullata. In that study, hydroxylation of tyrosine methyl ester was assayed indirectly by measuring oxygen consumption in the reaction. As expected for a tyrosinase-type enzyme, an initial lag phase was observed. A PO from Pacifastacus leniusculus (crayfish) hydroxylates tyrosine and another monophenol, tyramine (Aspan et al., 1995). It is likely that MsPO also has hydroxylating activity because incubation of MsPO with tyrosine generated oxidation products (detectable at 290 nm), and the formation of product over time included an initial lag phase; however, the zymogen form of MsPO was activated with a protease that was partially purified from M. sexta integument (a potential source of TH), and the integument fraction was not tested for hydroxylating activity (Aso et al., 1985). ProPO is expressed constitutively in specific types of hemocytes and is released into the hemolymph, presumably by hemocyte lysis (discussed in Ashida and Brey, 1997). The zymogen form of proPO is activated by a proPO activating proteinase, which cleaves proPO within the plasma (Kanost et al., 2004). Evidence that PO is involved in immune-related melanization comes from experiments in which inhibition of PO (by chemical inhibitors or RNA-mediated gene silencing) resulted in decreased melanization (Nappi, 1974; Liu et al., 1997; Shiao et al., 2001). It is less clear whether PO contributes to cuticle tanning. ProPO is transported from the hemolymph to the cuticle in Bombyx mori (Asano and Ashida, 2001); in addition, PO activity has been detected in the cuticles of other insect species. On the other hand, treatment of Calliphora vomitoria with an inhibitor of PO did not inhibit sclerotization of the puparium (Dennell, 1958), and RNAi-based silencing of PO in Tribolium castaneum had no detectable effect on cuticle tanning (Arakane et al., 2005). In addition to the hemolymph PO described above, M. sexta expresses a diphenoloxidase referred to as granular PO; this enzyme had no detectable hydroxylating activity and its known function is in cuticle pigmentation rather than cuticle tanning or immunity (Hiruma et al., 1985; Hiruma and Riddiford, 1988).
The goal of this study was to characterize MsTH and to assess the possible contribution of TH in the production of DOPA during cuticle biosynthesis and immune-related melanization in M. sexta. To characterize TH, we cloned a TH cDNA, purified the recombinant enzyme, and tested its activity. To discover what biological functions MsTH may have, we analyzed several expression profiles. To confirm that MsPO functions as a hydroxylase, we purified proPO from hemolymph, determined that the preparation did not contain TH, activated it with a detergent, and assayed for hydroxylating activity.
2. Materials and Methods
2.1. TH cDNA cloning
Degenerate primers (based on conserved regions of tyrosine hydroxylases) and cDNA from the integument of M. sexta larvae (in the fourth to fifth instar molt) were used to amplify a partial TH cDNA. 5′ and 3′ sequences were cloned by Rapid Amplification of cDNA Ends (RACE) using a GeneRacer kit (Invitrogen). Using primers encoding the start and stop codon regions, a cDNA encompassing the full coding region of TH was amplified from integument cDNAs, and the TH cDNA was ligated into the proExHTc expression vector (Life Technologies), which encodes an amino-terminal six histidine tag. Nucleotide sequences were confirmed by DNA sequencing of the full-length clone (GenBank accession no. EF592177).
Expression and purification of recombinant TH and production of TH antiserum
The XL1Blue strain of E. coli was transformed with the recombinant plasmid containing the TH cDNA. Expression trials demonstrated that rTH was insoluble when expression occurred at 37°C but was partially soluble when expression occurred at 18°C. To induce expression of TH in a 1 liter culture, the log phase culture at 37°C was cooled for 30 min at 18°C, then IPTG was added to a concentration of 1 mM, and the culture was incubated at 18°C and 125 rpm for 14 hours. Cells were collected by centrifugation and resuspended in lysis buffer (50 mM sodium phosphate, 300 mM NaCl, 10% glycerol, 0.5 mM DTT, 10 mM imidazole, pH 8). Cells were lysed by sonication, and a cleared lysate was obtained by centrifugation of the lysed cells. His-tagged protein was bound to Ni-NTA agarose (Qiagen) for 1 hour at 4°C with gentle agitation, then the slurry was poured into an empty 1.5 cm diameter column. Unbound proteins were removed by washing with lysis buffer that contained 20 mM imidazole. Bound proteins were eluted with lysis buffer containing 250 mM imidazole. Eluted proteins were concentrated with Microcon 30,000 MWCO units and applied to a Sephacryl 300 HR column (1.5 × 108 cm) at a rate of 0.3 ml/min. The column buffer was 50 mM sodium phosphate, 150 mM NaCl, 10% glycerol, 0.5 mM DTT, pH 6.8. The holoenzyme form of tyrosine hydroxylases is a tetramer, and most of the TH was detected in fractions with elution times consistent with the expected tetrameric mass (265 kDa). Six hundred μg purified TH (0.05 μg/μl) was stored in 50% column buffer, 50% glycerol at −20°C. Purification trials indicated a tendency of rTH to aggregate (only aggregate forms were detected after 1 month of storage); therefore, assays were done within 2 weeks of purification. Recombinant TH that was used to generate rabbit antibodies was purified in a similar manner, except that the gel filtation step was omitted. TH polyclonal antiserum was produced by Cocalico Biologicals.
2.2. Purification of proPO from hemolymph and proPO antiserum
Purification of proPO from hemolymph was done as described by Jiang et al. (1997), with one notable modification. During ammonium sulfate fractionation of hemolymph proteins, we retained a 38–48% saturated ammonium sulfate fraction rather than a 30–50% fraction. From 100 ml of larval hemolymph, we obtained 1.7 mg pure proPO. ProPO purified from M. sexta hemolymph contains two subunits. The polyclonal antiserum used for immunoblot analysis detects both of the subunits (Jiang et al., 1997).
2.3. Activity assays
Spectrophotometric assays were used to assess diphenoloxidase and tyrosine hydroxylase activity of PO. ProPO was activated with the detergent cetylpyridinium (CPC) at a concentration of 0.1% (Hall et al., 1995). Diphenoloxidase activity was determined by incubating 3 nM proPO with 0.1% CPC and 0.5 mM dopamine in 50 mM sodium phosphate, pH 6.5, and reading the absorbance at 475 nm for 2 minutes (n = 4). Linear regression was used to calculate the change in absorbance per second, and a molar extinction coefficient of 3058 M−1 cm−1 was used to estimate the rate of dopaminochrome formation (Baez et al., 1997). (Dopamine quinone is the product of dopamine oxidation by PO, but dopamine quinone is quickly converted to dopaminochrome through non-enzymatic reactions.) Tyrosine hydroxylase activity of PO was determined by a spectrophotometric assay and a radioassay (described below). Hydroxylase activity was determined by incubating 124 nM proPO with 0.1% CPC and 0.5 mM L-tyrosine in 50 mM sodium phosphate buffer, pH 6.5, and reading the absorbance at 280 nm for 6 minutes (n = 4). The reaction curves started with a variable lag phase followed by a linear phase. Linear regression was used to calculate the change in absorbance per second of the linear phase of each reaction, and then the mean of the four slopes was calculated. The average slope and a molar extinction coefficient of 1440 M−1 cm−1 were used to estimate the rate of DOPA formation (Duckworth and Coleman, 1970). Note that the DOPA formed in the reaction is expected to be oxidized further to DOPA quinone by PO, thus, DOPA is not the final product in the reaction, and products in addition to DOPA absorb light at 280 nm.
A tritiated-water release method was used to measure tyrosine hydroxylase activity of PO and TH (Reinhard et al., 1986). In these reactions, L-[3,5-3H]tyrosine is used as a substrate. 3H+ ions that are removed from the substrate during the hydroxylation reaction exchange with H+ ions in water leading to tritiated water. For PO assays, 31 nM proPO was incubated with 0.1% CPC, 50 μM L-tyrosine and 45 nM L-[3,5-3H]tyrosine (2.5 μCi/ml) in 50 mM sodium phosphate, pH 6.8. Control reactions did not include CPC. The 100 μl reactions were stopped by adding 1 ml 7.5% activated charcoal in 1 N HCl. Samples were centrifuged at 500 g for 10 minutes to pellet the charcoal containing adsorbed tritiated tyrosine, 250 μl of the supernatant containing tritiated water was added to 10 ml ScintiSafe 30%, and tritium content was measured by liquid scintillation counting. Reactions were done in triplicate. TH reactions were done essentially as described by Vie et al. (1999). Briefly, 19 nM TH was incubated with 50 μM L-tyrosine, 45 nM L-[3,5-3H]tyrosine (2.5 μCi/ml), 20 μM tetrahydrobiopterin, 10 uM ferrous ammonium sulfate, 15 mM β-mercaptoethanol, 0.07 mg/ml catalase, and 50 mM Hepes, pH 7.0. Activity was assayed as described (above) for the PO experiments.
2.4. Northern blot analysis
Total RNA was isolated from eggs and dissected tissues using the Ultraspec reagent method (Biotecx). The tissues analyzed were ventral nerve cord from day 1 fifth instar larvae; integument from larvae molting from the fourth to fifth instar; and fat body, hemocytes and midgut from day 2–4 fifth instar larvae. RNA (20 μg per lane) was fractionated in a 1% agarose, 6% formaldehyde gel and transferred to a nylon membrane by standard capillary blotting. A molecular mass ladder (Millenium Marker, Ambion) was included. After transfer, the membrane was stained with methylene blue to verify that a similar amount of RNA was loaded per lane. The full-length TH cDNA was labeled with 32P using the DecaPrimeII kit (Ambion). Hybridization was done at 60°C for 17 hours in 430 mM sodium phosphate, pH 7.2, 20 mM EDTA, 7% SDS, and 1% BSA. The blot was washed at 60°C with 2 × SSC, 0.1% SDS followed by 0.1 × SSC, 0.1% SDS. Two X-ray film exposure lengths (several hours or 3 days) were used to analyze hybridization results.
2.5. Immune induction
For analysis of TH and proPO RNA, fifth instar day 2 larvae were injected with 50 μl sterile water or 50 μl of 10 μg/μl Micrococcus luteus (Sigma) (n = 3 larvae for each treatment). Twenty-four hours after injection, fat body and hemocyte samples were collected from each larva. Total RNA was isolated using TRizol Reagent (Invitrogen). First-strand cDNA was synthesized with the use of a BD Sprint™ PowerScript™ PrePrimed Single Shots Kit including an oligo(dT) primer (Clontech). One μl of cDNA was used as the template for PCR reactions with primers specific for TH and 0.1 μl of cDNA was used as the template for PCR reactions with primers specific for proPO1, proPO2, and a control gene, ribososomal protein S3 (Rps3). Between 22 and 25 PCR cycles were used to produce DNA bands visible by ethidium bromide staining of agarose gels.
For analysis of TH and proPO protein, fifth instar day 2 larvae were injected with 100 μl of sterile 0.85% NaCl or 100 μl of 1 μg/μl M. luteus (n = 2 larvae for each treatment). Twenty-four hours after injection, fat body, hemocyte and plasma samples were collected and analyzed by immoblotting as described below.
2.6. Immunoblot analysis
Eggs in their first through fourth days of development were homogenized in TBS (25 mM Tris, 137 mM NaCl, 2.7 mM KCl, pH 7.4). To collect integument samples, larvae were dissected, and the gut, ventral nerve cord, fat body and some of the muscle was removed from the cuticle and epithelial layer. Integument from the first thoracic through second abdominal segments was obtained from three larvae that were in the process of molting from the fourth to fifth instar. Dorsal integument from the second thoracic, third thoracic and first abdominal segments was dissected from three prepupae (just prior to the metathoracic bar stage). Integuments were homogenized in TBS. Ventral nerve cords were dissected from three fifth instar day 0 larvae. They were rinsed with phosphate buffered saline and homogenized in 300 μl SDS-PAGE sample buffer. Fat body samples that had been dissected from fifth instar day 2 larvae or from control and immune induced larvae were rinsed with and homogenized in TBS. Hemolymph was obtained from day 2 fifth instar larvae by cutting the larval horn and collecting hemolymph into a chilled tube. For hemocyte and plasma samples, hemolymph was collected from control and immune induced larvae into equal volumes of anticoagulant saline, and the hemocytes were pelleted by centrifugation at 500 g for 15 min at 4°C. Hemocytes were homogenized in TBS. Midguts from three fifth instar day 2 larvae were rinsed with phosphate buffered saline and homogenized in TBS. For all tissue samples except ventral nerve cords, insoluble material was removed by centrifugation at 4°C, a small aliquot of the supernatant was saved for determining the concentration of soluble protein (using a Bradford method), and the remaining sample was immediately mixed with an equal volume of SDS-PAGE sample buffer and heated at 95°C. For the ventral nerve cord sample, the protein concentration was estimated based on the concentration of proteins in homogenates obtained by grinding the tissues in TBS instead of SDS-PAGE sample buffer. For the immunoblots shown in Figure 4B and 4C, 25 μg protein was loaded per lane. For the immunoblots shown in Figure 4E, 20 μg protein was loaded per lane for the blots incubated with the TH antiserum, and 4 μg was loaded per lane for the blots incubated with the proPO antiserum, except the blot of plasma proteins included 0.5 μl of plasma per lane. Standard methods were used for SDS-PAGE, protein transfer, and immunodetection.
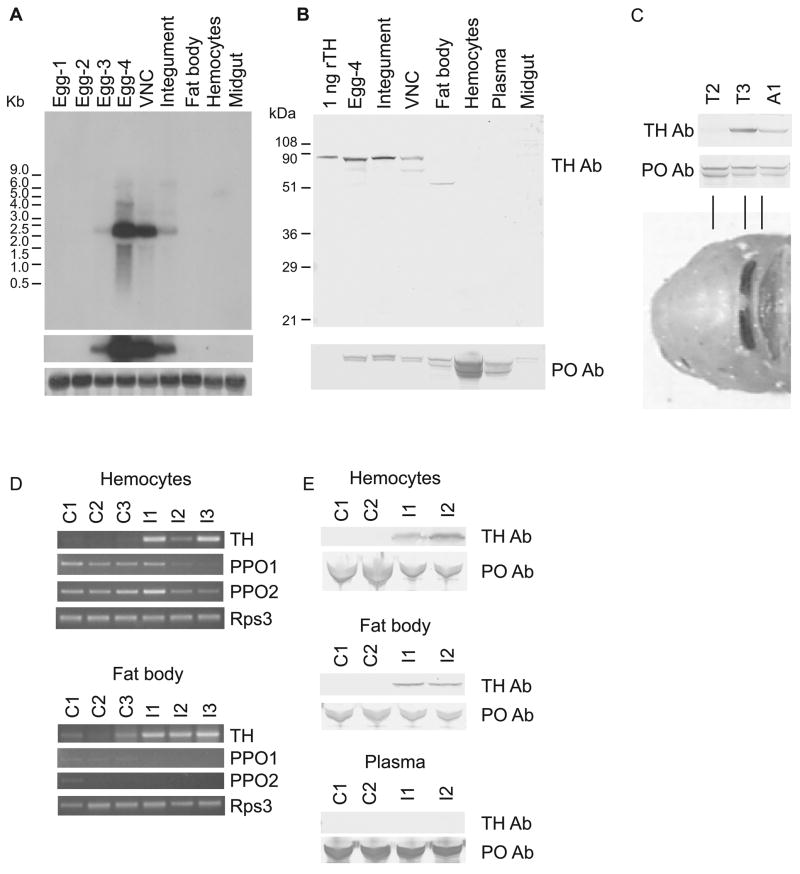
Figure 4. Expression of TH.
A) Northern blot analysis of TH demonstrates expression in eggs (day 3–4) and in larval ventral nerve cord and integument. Expression was not detected in fat body, hemocytes or midgut. Top panel: several hour exposure. Middle panel: 3 day exposure. Bottom panel: methylene blue staining of ribosomal RNA on blot demonstrating that similar amounts of RNA were loaded per lane. B) Immunoblot analysis of TH confirms expression in eggs, integument and ventral nerve cord. The lower molecular weight polypeptide in the VNC sample may be encoded by an alternatively spliced TH mRNA or may be a degradation product. The lower molecular weight polypeptide in the fat body sample is probably caused by the interaction of the polyclonal antiserum with an unknown protein because a second polyclonal antiserum generated against TH does not recognize this unknown protein (data not shown). Note that proPO (bottom panel) was abundant in hemocytes and present in plasma. The source of proPO detected in other tissues is likely to be adherent hemocytes. C) Immunoblot analysis illustrates higher abundance of TH in prepupal segments that were just starting to tan (third thoracic and first abdominal) compared with one that was not (second thoracic). Note that proPO was present in approximately equal amounts in each of these samples. The bottom panel shows the anterior part of a newly emerged pupa. The highly tanned metathoracic bar and the partially tanned first abdominal segment correlate with the presence of TH. D) Semiquantitative RT-PCR demonstrates expression of TH in the hemocytes and fat body of three larvae 24 hours after they were inoculated with bacteria (I1–3). TH PCR products from control larvae (C1-3) were barely detectable in this experiment in which 25 PCR cycles were performed. TH expression in the hemocytes and fat body of naive larvae was detectable when the number of PCR cycles was greater (not shown). PPO1 and PPO2 were not upregulated by the immune challenge. Rps3 was used as a template concentration control gene. E) Immunoblot analysis confirmed expression of TH in the hemocytes and fat body of infected larvae (I1-2) but not control larvae (C1-2). No TH was detected in plasma. In contrast, the concentration of proPO did not increase in response to inoculation.
3. Results
3.1 M. sexta tyrosine hydroxylase cDNA sequence
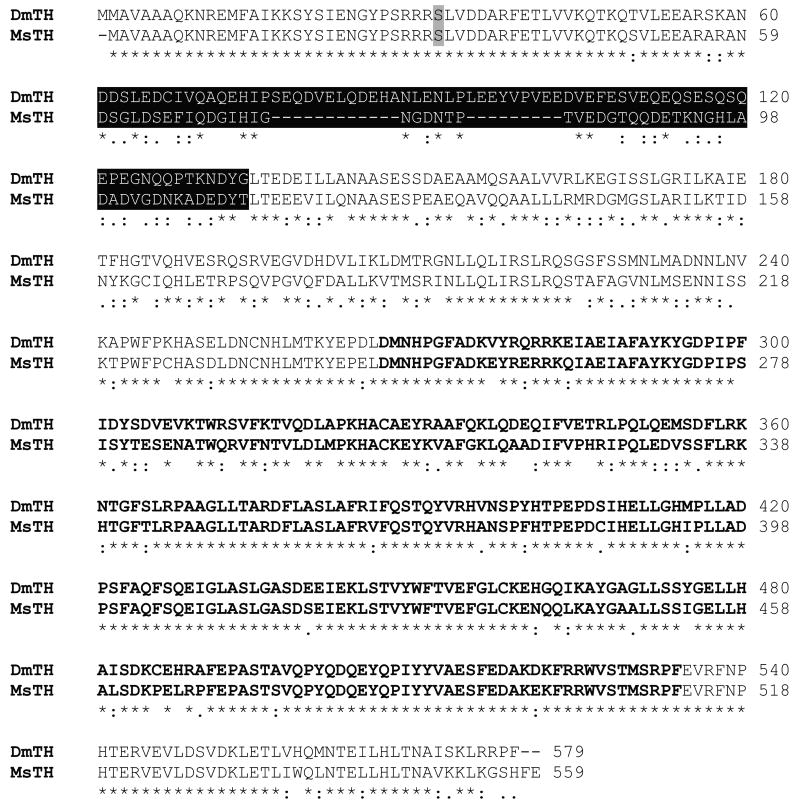
We cloned a 1,680 nucleotide cDNA encoding MsTH. The predicted polypeptide of 559 amino acids has a calculated molecular mass of 63.1 kDa and a predicted pI of 5.43 (Figure 1). RACE was used to identify 185 bases of the 5′ untranslated region (UTR) and 390 bases of the 3′ UTR; however, the lack of a polyA signal in the 3′ sequence suggests that the 3′ UTR is actually greater than 390 bases. The predicted amino acid sequence is 70% identical to the epidermal (long) form of TH from D. melanogaster (DmTH). Like the epidermal form of DmTH, the MsTH sequence includes an acidic region (pI = 3.66) encoded by two exons (data not shown). The activity of vertebrate THs and DmTH is regulated by cAMP-dependent protein kinases, which phosphorylate one or more serine residues (Ser32 in DmTH) (Vie et al., 1999). The MsTH sequence includes a conserved serine (Ser31) that may be a target for phosphorylation. The MsTH sequence also contains a conserved C-terminal region that is probably required for tetramer formation (Goodwill et al., 1997).
Figure 1. Amino acid alignment of MsTH and DmTH.
An alignment of the predicted amino acid sequence of MsTH with the epidermal form of DmTH is shown. DmTH Ser32 (shaded) is phosphorylated by cAMP-dependent protein kinase and is conserved in MsTH (Ser31). Residues highlighted in black are encoded by two alternatively spliced exons. Residues in bold comprise the putative catalytic domain. The carboxy-terminal region (in standard type) is predicted to be a tetramerization domain. (The putative domain boundaries were selected based on the experimentally determined domain boundaries of rat TH [Goodwill et al., 1997].)
3.2 Assay and comparison of MsTH and PO catalytic activity
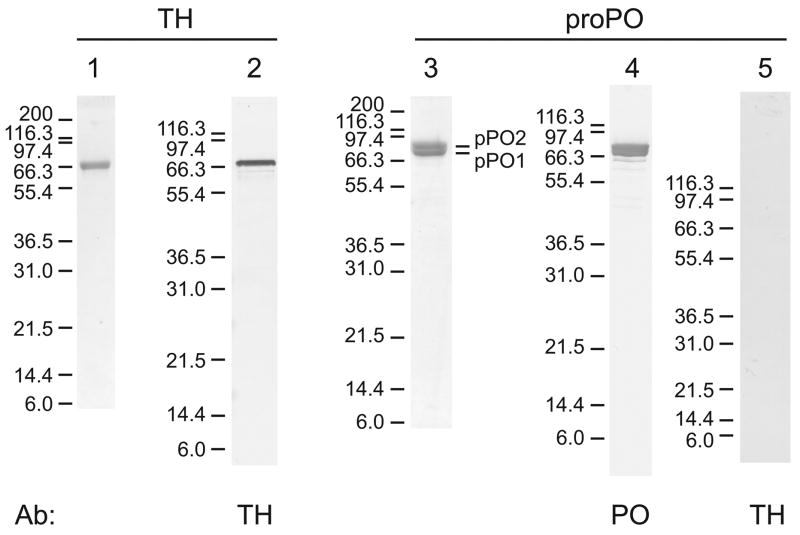
Recombinant TH containing an amino-terminal six-His tag was expressed in E. coli, and purified by nickle affinity chromatography. Because the polypeptides tended to form aggregates, the tetrameric form of rTH was purified by gel filtration. SDS-PAGE analysis confirmed that the enzyme was highly purified (Figure 2, lane 1). Rabbit antibodies were generated against the recombinant protein, and the resulting antiserum was used to detect 10 ng of TH by immunoblot analysis (Figure 2, lane 2).
Figure 2. SDS-PAGE and immunoblot analysis of purified recombinant TH and native proPO.
Recombinant TH was analyzed by Coomassie staining (0.5 μg, lane 1) or detection with anti-TH antiserum (10 ng, lane 5). The predicted mass of the monomer form of rTH is 66.3 kDa. 2 μg proPO was analyzed by Coomassie staining (lane 3) or detection with anti-proPO antiserum (lane 4) or anti-TH antiserum (lane 5). ProPO subunits 1 and 2, which have predicted masses of 79 and 80 kDa, respectively, were partially resolved. Note that no TH was detected in the proPO fraction. Molecular mass standards (in kDa) are indicated to the left of each lane.
ProPO was purified from M. sexta larval hemolymph. SDS-PAGE analysis demonstrated that the proPO fraction contained proPO subunits 1 and 2 and that the proPO was very pure (Figure 2, lane 3). Significantly, no TH could be detected by immunoblot analysis in a 2 μg sample of proPO (Figure 2, lane 5).
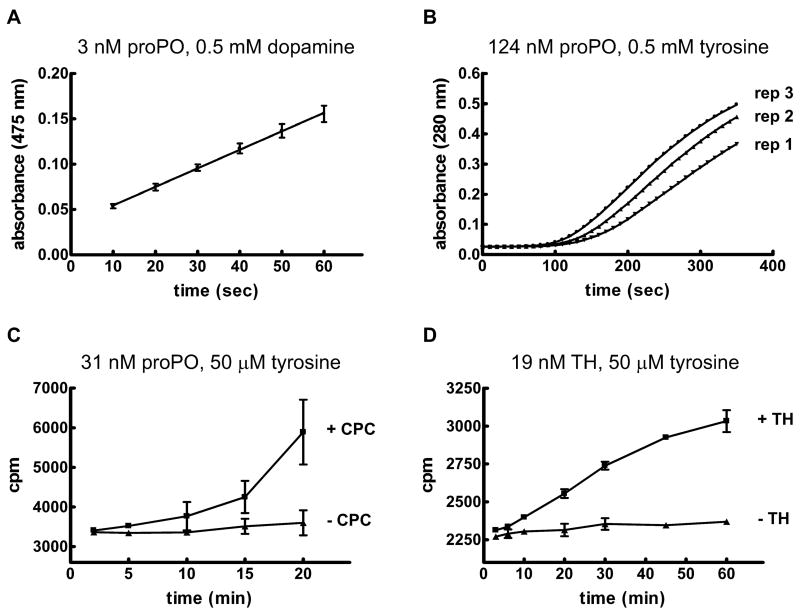
The enzymatic activities of hemolymph PO and rTH were assayed. For these experiments, proPO was activated with a detergent (0.1% CPC). A standard spectrophotometric assay for diphenoloxidase activity was used to estimate a reaction rate of 80 nmoles dopaminochrome formed min−1 μg−1 when the substrate was 0.5 mM dopamine and the concentration of proPO was 3 nM (Figure 3A). The estimated reaction rate for hydroxylase activity was much lower at 4 nmoles DOPA formed min−1 μg−1 when the substrate was 0.5 mM tyrosine and the concentration of proPO was 124 nM (Figure 3B). In addition, activity was observed only after a variable lag phase. A tritiated-water release method that is a more specific assay for hydroxylation was also utilized. The substrate concentration in these reactions was low (50 μM tyrosine), which resulted in low activity. It was not possible to quantify the reaction rate because the relationship between the amount of product formed over time was not linear; nevertheless, the results of this experiment demonstrate that hydroxylation of tyrosine occurred during the reaction (Figure 3C). The tritiated water-release method was also used to verify hydroxylation activity of rTH (Figure 3D). The reaction rate was estimated to be 2 pmoles DOPA formed min−1 μg−1 when the substrate was 0.5 mM tyrosine and the concentration of TH was 19 nM. This rate is lower than those observed for the DmTH isoforms and for rat TH (Vie et al., 1999; Ribeiro et al., 1992), and we expect that problems with aggregation and/or the presence of an amino-terminal His tag may be responsible for the low activity.
Figure 3. Tyrosine hydroxylase activity of MsPO and MsTH.
A) 3 nM proPO was incubated with 0.1% CPC (to activate proPO) and 0.5 mM dopamine in 50 mM sodium phosphate, pH 6.5. Formation of dopaminochrome was detected as an increase in absorbance at 475 nm. Data are expressed as mean ± SD (n = 4). B) 124 nM proPO was incubated with 0.1% CPC and 0.5 mM tyrosine in 50 mM sodium phosphate, pH 6.5. Dopa formation was detected as an increase in absorbance at 280 nm. The results of three replicates are shown. C) 31 nM proPO was incubated in reaction buffer containing 50 μM tyrosine and 45 nM 3H-tyrosine, with or without 0.1% CPC. Tyrosine hydroxylase activity was assessed by quantifying the formation of tritiated water. Data are expressed as mean ± SD (n = 3). Note that the large standard deviations in the observed values were due to the variable lag phase of PO activity. D) 19 nM TH was incubated in reaction buffer containing 50 μM tyrosine and 45 nM 3H-tyrosine. Control reactions did not contain TH. Activity was assessed by quantifying the formation of tritiated water. Data are expressed as mean ± SD (n = 3).
3.3 Expression profiles of TH and PPO
To assess possible functions of TH in M. sexta, we studied TH expression profiles. TH mRNA was present in eggs (Figure 4A) at the stage when tanning of the mandibles, legs, setae, spiracles and horn occurs (Dorn et al., 1987). TH mRNA was also detected in the integument of larvae molting from the fourth to the fifth instar and in the ventral nerve cord but not fat body, hemocytes or midgut of feeding stage fifth instar larvae (Figure 4A). Immunoblot analysis verified the presence of TH in late stage eggs, the integument of molting larvae and the ventral nerve cord (Figure 4B). The TH antiserum detected polypeptides of two molecular masses in the ventral nerve cord. The smaller form may be encoded by an alternatively spliced TH mRNA (a smaller form of TH is present in D. melanogaster neural cells [Birman et al., 1994]), or TH may have been partially degraded during sample collection and preparation. TH was not detected in the fat body, hemocytes, plasma or midgut of naive larvae (Figure 4B). The TH expression pattern differed considerably from that of proPO (Figure 4B). As expected from the results of a previous study (Jiang et al., 1997), hemocytes were the main source of proPO, and proPO was present in the plasma. Small amounts of proPO were observed in all other tissue samples, which was not surprising because hemocytes adhere to many tissues (discussed in Kim et al., 2005). An immunoblot analysis demonstrated that TH was more abundant in the integument of two prepupal segments that were just starting to tan compared with one that was not (Figure 4C). In contrast, proPO was present in approximately equivalent amounts in each of the prepupal segements.
TH mRNA was not detected by northern blot analysis in the hemocytes or fat body of healthy larvae (Figure 4A); however, evidence of TH expression in these tissues was suggested by a more sensitive method, RT-PCR (data not shown). To test for induction of expression in these cell types following an immune challenge, we inoculated three larvae with M. luteus and injected three control larvae with sterile water, and then used RT-PCR to evaluate expression. TH mRNA was present in the hemocytes and fat body of the three infected individuals 24 hours post infection, and a very low level of TH mRNA was present in the control larvae (Figure 4D). Upregulation of TH in response to infection was corroborated by immunoblot results demonstrating TH in the fat body and hemocytes of infected larvae (Figure 4E). TH was not detected in plasma, an expected result given that TH does not contain a signal peptide. Upregulation of the proPO1 and proPO2 genes was not observed (Figure 4D), and proPO remained at a high, constitutive level in plasma following the immune challenge (Figure 4E).
Discussion
In this study, we characterized a cDNA that encodes MsTH, demonstrated that MsTH and MsPO have different tissue specificities and different responses to immune challenge, and verified that MsTH and MsPO can hydroxylate tyrosine. The MsTH cDNA, which was cloned from integument mRNA, is highly similar to the epidermal form of DmTH, which contains an acidic region that is lacking in the alternatively spliced neural form of DmTH. The acidic region has been shown to make the epidermal form of DmTH more constitutively active and less sensitive to feedback inhibition by dopamine, two properties that would improve TH function during cuticle tanning (Vie et al., 1999). A correlation between the amount of MsTH expression in the integument and the degree of cuticle tanning (in eggs and prepupal integument) suggests that at least some of the DOPA required for cuticle tanning is generated by TH. Expression of MsTH in integument and ventral nerve cord is consistent with DmTH mutant phenotypes, namely, decreased cuticle tanning and behavioral abnormalities (Budnik and White, 1987; Neckameyer and White, 1993; Neckameyer, 1996; True et al., 1999; Friggi-Grelin et al., 2003). Our results are also consistent with those of a previous study that used a heterologous antibody to identify MsTH in H-cells in the ventral nerve cord (Mesce et al., 2001).
Upregulation of MsTH expression in the hemocytes and fat body of infected larvae indicates a likely immune function for TH. This role was suggested by two previous discoveries: the presence of TH cDNA in a pool of immune induced cDNAs from the hemocytes of G. mellonella (Seitz et al., 2003) and microarray data that suggested slight upregulation (~ 2 fold) of TH in D. melanogaster in response to septic injury (De Gregorio et al., 2001). However, other microarray studies of immune related genes in insects have not detected upregulation of TH (Oduol et al., 2000; Irving et al., 2001; Aquilar et al., 2005). In all of these microarray studies, RNAs from whole insects were analyzed. One explanation for the failure of three of the studies to detect TH as immune induced is that constitutive expression in epidermal cells may have obscured upregulation in hemocytes and fat body. We observed no significant difference in TH transcript abundance in whole naive larvae compared with whole inoculated larvae (data not shown) despite clearly increased transcript abundance in hemocytes and fat body cells. In contrast to TH expression, neither of the genes that encode the proPO subunits were upregulated in response to injection of M. luteus, and the concentration of proPO did not increase in hemocytes, fat body or plasma following the immune challenge. This result is not surprising considering that proPO is abundant in the hemolymph of naive larvae and thus is constitutively available for an immediate immune response. These results differ from those of Eleftherianos et al (2007), who reported increased expression of proPO-2 in fat body and hemocytes of M. sexta after injection of bacteria. However, we consistently observe constitutive expression of both proPO genes in hemocytes, with little if any expression in fat body (Jiang et al., 1997), and a lack of upregulated expression after immune challenge (Figure 4 and data not shown).
We have confirmed that both MsPO and MsTH have the ability to hydroxylate tyrosine and therefore may contribute to the pool of DOPA that is essential for various physiological processes. Based on studies of other insect species, our working model had been that PO functions in immunity, whereas TH functions in cuticle tanning. The reasons for this assumption are cited in the Introduction section: PO is required for immune associated melanization (Nappi, 1974; Liu et al., 1997; Shiao et al., 2001) but, at least in T. castaneum, PO is not essential for cuticle tanning (Arakane et al., 2005); in contrast, TH is required for cuticle tanning in D. melanogaster (Budnik and White, 1987; Neckameyer and White, 1993; True et al., 1999; Friggi-Grelin et al., 2003), but no clear requirement for TH in immunity has been demonstrated. Although we have not demonstrated conclusively that MsTH has an immune function, immune induction of MsTH in hemocytes and fat body strongly suggests such a role. This finding leads us to the question: if both MsTH and MsPO are involved in immune-associated melanin synthesis, what are their specific contributions to the supply of DOPA during an immune response? We would like to propose a testable but still hypothetical model in which MsPO and MsTH have separate functions (Figure 5).
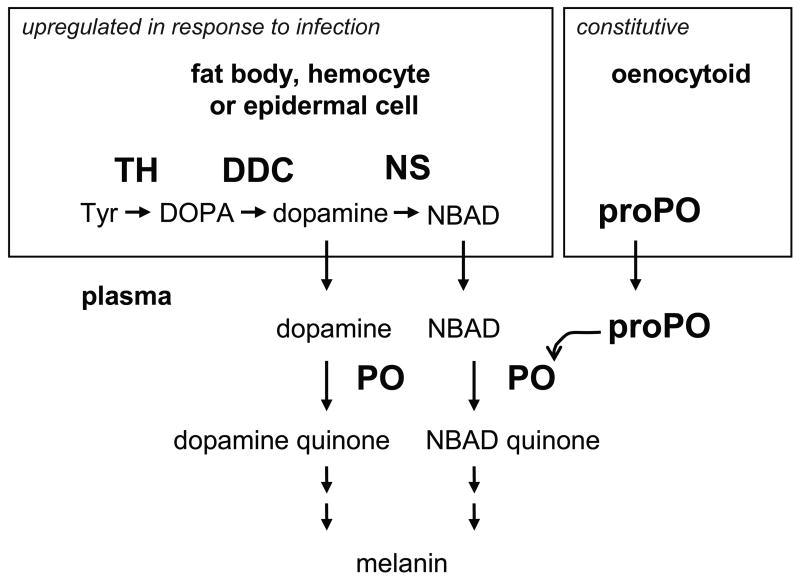
Figure 5. Model of melanin synthesis in response to infection in M. sexta.
In fat body, hemocytes and/or epidermal cells, dopamine is produced from tyrosine. TH hydroxylates tyrosine to DOPA, dopa decarboxylase (DDC) decarboxylates DOPA to dopamine, and dopamine is transported to the hemolymph. This process is upregulated in response to immune challenge. Some of the dopamine is converted to NBAD by NBAD synthase (NS) and then transported to the hemolymph. ProPO is constitutively expressed in oenocytoids and is released into the plasma via lysis of oenocytoids. ProPO is activated by a serine protease cascade, and PO oxidizes dopamine and NBAD to produce dopamine quinone and NBAD quinone. Further reactions (not shown) lead to the formation of melanin.
Our model differs from the one most often mentioned in papers describing insect immune processes, although it is similar to one by De Gregorio et al. (2001). Most immune-related melanization models begin with the hydroxylation of tyrosine by PO to produce DOPA, then some of the DOPA is thought to be oxidized by PO to generate DOPA quinone, and some is thought to be decarboxylated by dopa decarboxylase (DDC) to produce dopamine, which is then oxidized by PO to dopamine quinone. We have revised this model (Figure 5) based on the results of previous studies of tyrosinase activity, PO activity, and the immune function of DDC (see below) and on the data presented in our study (namely that MsPO has a much higher diphenoloxidase activity than hydroxylating activity and that MsTH is likely to have an immune function). We propose that dopamine is synthesized from tyrosine by a pathway of two intracellular enzymes, TH and DDC, (in fat body, hemocytes and epidermal cells). TH hydroxylates tyrosine to DOPA and DDC decarboxylates DOPA to dopamine. Transport of dopamine into the hemolymph would allow it to be used as a substrate for PO, which is present in plasma and activated in response to infection. Dopamine quinone would undergo subsequent reactions leading to melanin formation. In addition, it is likely that some of the dopamine that is synthesized is converted to N-β-alanyldopamine (NBAD) by NBAD synthase and that NBAD is oxidized by PO to generate NBAD quinone (Kim et al., 2000). The following observations support this model.
Although both TH and PO can hydroxylate tyrosine, TH appears to be a better candidate for producing significant amounts of DOPA from tyrosine. This idea is based on the assumption that the mechanism of PO activity is similar to that of mammalian tyrosinase, and on the fact that DOPA is the final product of TH activity but is a minor product of tyrosinase activity. Models of tyrosinase (and PO) activity often depict DOPA as a product of the hydroxylation reaction, but studies of tyrosinase activity have yet to clarify whether DOPA is released from the active site after the hydroxylation step or whether DOPA is simply an intermediate in the generation of DOPA quinone (Garcia-Borron and Solano, 2002). If it is the latter case, it is obvious that the hydroxylation reaction should generate very little DOPA. If it is the former, we would still expect DOPA accumulation to be low because the kcat for diphenoloxidase activity of tyrsosinase is much higher than the kcat of hydroxylating activity; thus, most of the DOPA that is formed by tyrosinase should be oxidized quickly to DOPA quinone (Rodriguez-Lopez et al., 1992). DOPA also can be produced from DOPA quinone by the following mechanism: spontaneous endocyclic ring formation produces cyclodopa, and cyclodopa is oxidized by DOPA quinone to generate DOPA and dopachrome; however, the DOPA that is formed by this mechanism is rapidly oxidized by tyrosinase (Riley, 1993).
The melanin that is formed during insect immune responses appears to be derived more from dopamine than from DOPA. This observation is based on three studies that demonstrate a requirement for DDC in immune type melanin production (Nappi et al., 1992; Liu et al., 1997; Huang et al., 2005). In the first study, a strain of D. melanogaster that had a temperature sensitive allele of DDC demonstrated a striking reduction in the melanization of parasitoid eggs at the restrictive temperature. In the other two studies, inhibition of DDC activity by a chemical inhibitor or an anti-sense RNA technique dramatically increased the amount of time required for melanin to form on the surface of filarial worms exposed to the hemolymph of Armigeres subalbatus (for example, in vitro incubation of filarial worms with hemolymph resulted in visible melanin formation after 3 minutes in the absence of a DDC inhibitor and after 3 hours in the presence of the DDC inhibitor). If the normal function of PO is to hydroxylate tyrosine and oxidize DOPA leading to DOPA quinone (and subsequent melanin formation), then a reduction in DDC activity should not have such a large negative effect on melanization. In contrast, if PO must oxidize dopamine (or a dopamine derivative such as NBAD) to generate a normal melanization response, then a reduction in DDC activity should have a major effect on melanization. The importance of DDC in immunity is supported by several studies demonstrating that DDC is upregulated after an immune challenge, for example, in Tenebrio molitor (Kim et al., 2000), D. melanogaster (De Gregorio et al., 2001), and the fat body of M. sexta larvae (Zhu et al., 2003). NBAD synthase is also upregulated after immune challenge (Kim et al., 2000; Schachter et al., 2007).
Compared with dopamine and NBAD, DOPA is a poor substrate for PO. For example, the Km for oxidation of dopamine by MsPO is ~6 times lower than the Km for DOPA, and the Km for NBAD is ~10 times lower than the Km for DOPA (Aso et al., 1985). In addition, the hemolymph of M. sexta larvae contains detectable concentrations of dopamine and NBAD but not DOPA (Hopkins et al., 1984). Data on the substrate specificity of insect POs, the availability of potential PO substrates, and the poor solubility of DOPA led Sugumaran (2002) to suggest that dopamine and dopamine derivatives such as NBAD, rather than DOPA, are the natural substrates of insect POs.
It is likely that the model proposed here is an oversimplification of the actual biochemical pathways that occur during the synthesis of melanin in response to infection; however, we think that the major steps in the pathway shown in Figure 5 are well supported by the currently available data. Future experiments using knock down methods and analysis of hemolymph catechol metabolism will allow further testing and refinement of the model.
Acknowledgments
We thank Dr. Karl Kramer for helpful suggestions regarding this work and this manuscript. We thank Neal Dittmer for the photograph of a newly emerged Manduca pupa. This is contribution 07-254-J from the Kansas Agricultural Experiment Station. This work was supported by NIH grant GM41247.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aquilar R, Jedlicka AE, Mintz M, Mahairaki V, Scott AL, Dimopoulos G. Global gene expression analysis of Anopheles gambiae responses to microbial challenge. Insect Biochem Mol Biol. 2005;35:709–719. doi: 10.1016/j.ibmb.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Arakane Y, Muthukrishnan S, Beeman RW, Kanost MR, Kramer KJ. Laccase 2 is the phenoloxidase gene required for beetle cuticle tanning. Proc Natl Acad Sci USA. 2005;102:11337–11342. doi: 10.1073/pnas.0504982102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano T, Ashida M. Cuticular pro-phenoloxidase of the silkworm, Bombyx mori: purification and demonsration of its transport from hemolymph. J Biol Chem. 2001;14:11100–11112. doi: 10.1074/jbc.M008426200. [DOI] [PubMed] [Google Scholar]
- Ashida M, Brey PT. Recent advances in research on the insect prophenoloxidase cascade. In: Brey PT, Hultmark D, editors. Molecular Mechanisms of Immune Responses in Insects. Chapman & Hall; London: 1997. pp. 133–172. [Google Scholar]
- Aso Y, Kramer KJ, Hopkins TL, Lookhart GL. Characterization of hemolymph protyrosinase and a cuticular activator from Manduca sexta (L.) Insect Biochem. 1985;15:9–17. [Google Scholar]
- Aspan A, Huang TS, Cerenius L, Soderhall K. cDNA cloning of prophenoloxidase from the freshwater crayfish Pacifastacus leniusculus and its activation. Proc Natl Acad Sci USA. 1995;92:939–943. doi: 10.1073/pnas.92.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baez S, Segura-Aguilar J, Widersten M, Johansson AS, Mannervik B. Glutathione transferases catalyze the detoxification of oxidized metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochem J. 1997;324:25–28. doi: 10.1042/bj3240025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birman S, Morgan B, Anzivino M, Hirsh J. A novel and major isoform of tyrosine hydroxylase in Drosophila is generated by alternative RNA processing. J Biol Chem. 1994;269:26559–26567. [PubMed] [Google Scholar]
- Budnick V, White K. Genetic dissection of dopamine and serotonin synthesis in the nervous system of Drosophila melanogaster. J Neurogenet. 1987;4:309–314. [PubMed] [Google Scholar]
- Chase MR, Raina K, Bruno J, Sugumaran M. Purification, characterization and molecular cloning of prophenoloxidases from Sarcophaga bullata. Insect Biochem Mol Biol. 2000;30:953–967. doi: 10.1016/s0965-1748(00)00068-0. [DOI] [PubMed] [Google Scholar]
- De Gregorio E, Spellman PT, Rubin GM, Lemaitre B. Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc Natl Acad Sci USA. 2001;98:12590–12595. doi: 10.1073/pnas.221458698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennell R. The amino acid metabolism of a developing insect cuticle: the larval cuticle and puparium of Calliphora vomitoria III. The formation of the puparium. Proc R Soc Lond B Biol Sci. 1958;149:176–183. doi: 10.1098/rspb.1958.0060. [DOI] [PubMed] [Google Scholar]
- Dorn A, Bishoff ST, Gilbert LI. An incremental analysis of the embryonic development of the tobacco hornworm, Manduca sexta. Int J Invertebr Reprod Dev. 1987;11:137–158. [Google Scholar]
- Duckworth HW, Coleman JE. Physiochemical and kinetic properties of mushroom tyrosinase. J Biol Chem. 1970;245:1613–1625. [PubMed] [Google Scholar]
- Eleftherianos I, Boundy S, Joyce SA, Aslam S, Marshall JW, Cox RJ, Simpson TJ, Clarke DJ, ffrench-Constant RH, Reynolds SE. An antibiotic produced by an insect-pathogenic bacterium suppresses host defenses through phenoloxidase inhibition. Proc Natl Acad Sci USA. 2007;104:2419–2424. doi: 10.1073/pnas.0610525104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friggi-Grelin F, Coulom H, Meller M, Gomez D, Hirsh J, Birman S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol. 2003;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- Futahashi R, Fujiwara H. Melanin-synthesis enzymes coregulates stage-specific larval cuticular markings in the swallowtail butterfly, Papilio xuthus. Dev Genes Evol. 2005;215:519–529. doi: 10.1007/s00427-005-0014-y. [DOI] [PubMed] [Google Scholar]
- Garcia-Borron JC, Solano F. Molecular anatomy of tyrosinase and its related proteins: beyond the histidine-bound metal catalytic center. Pigment Cell Res. 2002;15:162–173. doi: 10.1034/j.1600-0749.2002.02012.x. [DOI] [PubMed] [Google Scholar]
- Goodwill KE, Sabatier C, Marks C, Raag R, Fitzpatrick PF, Stevens RC. Crystal structure of tyrosine hydroxylase at 2.3 angstroms and its implications for inherited neurodegenerative diseases. Nat Struct Biol. 1997;4:578–585. doi: 10.1038/nsb0797-578. [DOI] [PubMed] [Google Scholar]
- Hall M, Scott T, Sugumaran M, Soderhall K, Law JH. Proenzyme of Manduca sexta phenol oxidase: purification, activation, substrate specificity of the active enzyme, and molecular cloning. Proc Natl Acad Sci USA. 1995;92:7764–7768. doi: 10.1073/pnas.92.17.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruma K, Riddiford LM. Granular phenoloxidase involved in cuticular melanization in the tobacco hornworm: regulation of its synthesis in the epidermis by juvenile hormone. Dev Biol. 1988;130:87–97. doi: 10.1016/0012-1606(88)90416-2. [DOI] [PubMed] [Google Scholar]
- Hiruma K, Riddiford LM, Hopkins TL, Morgan TD. Roles of dopa decarboxylase and phenoloxidase in the melanization of the tobacco hornworm and their control by 20-hydroxyecdysone. J Comp Physiol B. 1985;155:659–669. doi: 10.1007/BF00694579. [DOI] [PubMed] [Google Scholar]
- Hopkins TL, Morgan TD, Kramer KJ. Catecholamines in haemolymph and cuticle during larval, pupal and adult development of Manduca sexta (L.) Insect Biochem. 1984;14:533–540. [Google Scholar]
- Huang CY, Chou SY, Bartholomay LC, Christensen BM, Chen CC. The use of gene silencing to study the role of dopa decarboxylase in mosquito melanization reactions. Insect Mol Biol. 2005;14:237–244. doi: 10.1111/j.1365-2583.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- Irving P, Troxler L, Heuer TS, Belvin M, Kopczynski C, Reichart JM, Hoffmann JA, Hetru C. A genome-wide analysis of immune responses in Drosophila. Proc Natl Acad Sci USA. 2001;98:15119–15124. doi: 10.1073/pnas.261573998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Wang Y, Ma C, Kanost MR. Subunit composition of pro-phenol oxidase from Manduca sexta: molecular cloning of subunit proPO-P1. Insect Biochem Mol Biol. 1997;27:835–850. doi: 10.1016/s0965-1748(97)00066-0. [DOI] [PubMed] [Google Scholar]
- Kanost MR, Jiang H, Yu XQ. Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol Rev. 2004;198:97–105. doi: 10.1111/j.0105-2896.2004.0121.x. [DOI] [PubMed] [Google Scholar]
- Kim MH, Joo CH, Cho MY, Kwon TH, Lee KM, Natori S, Lee TH, Lee BL. Bacterial-injection-induced syntheses of N-β-alanyldopamine and dopa decarboxylase in the hemolymph of coleopteran insect, Tenebrio molitor larvae. Eur J Biochem. 2000;267:2599–2608. doi: 10.1046/j.1432-1327.2000.01271.x. [DOI] [PubMed] [Google Scholar]
- Kim SR, Yao R, Han Q, Christensen BM, Li J. Identification and molecular characterization of a prophenoloxidase involved in Aedes aegypti chorion melanization. Insect Mol Biol. 2005;14:185–194. doi: 10.1111/j.1365-2583.2004.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner A, Pawelek J. Mammalian tyrosinase catalyzes three reactions in the biosynthesis of melanin. Science. 1982;217:1163–1165. doi: 10.1126/science.6810464. [DOI] [PubMed] [Google Scholar]
- Liu CT, Hou RF, Ashida M, Chen CC. Effects of inhibitors of serine protease, phenoloxidase and dopa decarboxylase on the melanization of Dirofilaria immitis microfilariae with Armigeres subalbatus haemolymph in vitro. Parasitology. 1997;115:57–68. doi: 10.1017/s0031182097001108. [DOI] [PubMed] [Google Scholar]
- Mesce KA, DeLorme AW, Frelje TC, Klukas KA. Dopamine-synthesizing neurons include the putative H-cell homologue in the moth Manduca sexta. J Comp Neurol. 2001;430:501–517. doi: 10.1002/1096-9861(20010219)430:4<501::aid-cne1046>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Molina FG, Munoz JL, Varon R, Rodriguez Lopez JN, Canovas FG, Tudela J. An approximate analytical solution to the lag period of monophenolase activity of tyrosinase. Int J Biochem Cell Biol. 2007;39:238–252. doi: 10.1016/j.biocel.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Nappi AJ. The role of melanization in the immune reaction of larvae of Drosophila algonquin against Pseudeucoila bochei. Parasitology. 1974;66:23–32. [Google Scholar]
- Nappi AJ, Carton Y, Li J, Vass E. Reduced cellular immune competence of a temperature-sensitive dopa decarboxylase mutant strain of Drosophila melanogaster against the parasite Leptopilina boulardi. Comp Biochem Physiol B. 1992;101:453–460. doi: 10.1016/0305-0491(92)90027-o. [DOI] [PubMed] [Google Scholar]
- Neckameyer WS. Multiple roles for dopamine in Drosophila development. Dev Biol. 1996;176:209–219. doi: 10.1006/dbio.1996.0128. [DOI] [PubMed] [Google Scholar]
- Neckameyer WS, White K. Drosophila tyrosine hydroxylase is encoded by the pale locus. J Neurogenet. 1993;8:189–199. doi: 10.3109/01677069309083448. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Hayakawa Y. Insect cytokine, growth-blocking peptide, is a primary regulator of melanin-synthesis enzymes in armyworm larval cuticle. FEBS J. 2007;274:1768–1777. doi: 10.1111/j.1742-4658.2007.05724.x. [DOI] [PubMed] [Google Scholar]
- Oduol F, Xu J, Niare O, Natarajan R, Vernick KD. Genes identified by an expression screen of the vector mosquito Anopheles gambiae display differential molecular immune response to malaria parasites and bacteria. Proc Natl Acad Sci USA. 2000;97:11397–11402. doi: 10.1073/pnas.180060997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz SH. The tyrosine hydroxylase activity of mammalian tyrosinase. J Biol Chem. 1966;241:161–168. [PubMed] [Google Scholar]
- Ribeiro P, Wang Y, Citron BA, Kaufman S. Regulation of recombinant rat tyrosine hydroxylase by dopamine. Proc Natl Acad Sci USA. 1992;89:9593–9597. doi: 10.1073/pnas.89.20.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard JF, Smith GK, Nichol CA. A rapid and sensitive assay for tyrosine-3-monooxygenase based upon the release of 3H2O and adsorption of [3H]-tyrosine by charcoal. Life Sci. 1986;39:2185–2189. doi: 10.1016/0024-3205(86)90395-4. [DOI] [PubMed] [Google Scholar]
- Riley PA. Mechanistic aspects of the control of tyrosinase activity. Pigment Cell Res. 1993;6:182–185. doi: 10.1111/j.1600-0749.1993.tb00600.x. [DOI] [PubMed] [Google Scholar]
- Rodriquez-Lopez JN, Tudela J, Varon R, Garcia-Carmona F, Garcia-Canovas F. Analysis of a kinetic model for melanin biosynthesis pathway. J Biol Chem. 1992;267:3801–3810. [PubMed] [Google Scholar]
- Schachter J, Pérez MM, Quesada-Allué LA. The role of N-β-alanyldopamine synthase in the innate immune response of two insects. J Insect Physiol. 2007 doi: 10.1016/j.jinsphys.2007.06.010. In press. [DOI] [PubMed] [Google Scholar]
- Seitz V, Clermont A, Wedde M, Hummel M, Vilcinskas A, Schlatterer K, Podsiadlowski L. Identification of immunorelevant genes from greater wax moth (Galleria mellonella) by a subtractive hybridization approach. Dev Comp Immunol. 2003;27:207–215. doi: 10.1016/s0145-305x(02)00097-6. [DOI] [PubMed] [Google Scholar]
- Shiao SH, Higgs S, Adelman Z, Christensen BM, Liu SH, Chen CC. Effect of prophenoloxidase expression knockout on the melanization of microfilariae in the mosquito Armigeres subalbatus. Insect Mol Biol. 2001;10:315–321. doi: 10.1046/j.0962-1075.2001.00268.x. [DOI] [PubMed] [Google Scholar]
- Sugumaran M. Comparative biochemistry of eumelanogenesis and the protective roles of phenoloxidase and melanin in insects. Pigment Cell Res. 2002;15:2–9. doi: 10.1034/j.1600-0749.2002.00056.x. [DOI] [PubMed] [Google Scholar]
- True JR, Edwards KA, Yamamoto D, Carroll SB. Drosophila wing melanin patterns form by vein-dependent elaboration of enzymatic prepatterns. Curr Biol. 1999;9:1382–1391. doi: 10.1016/s0960-9822(00)80083-4. [DOI] [PubMed] [Google Scholar]
- Ulrich R, Hofrichter M. Enzymatic hydroxylation of aromatic compounds. Cell Mol Life Sci. 2007;64:271–293. doi: 10.1007/s00018-007-6362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vie A, Cigna M, Toci R, Birman S. Differential regulation of Drosophila tyrosine hydroxylase isoforms by dopamine binding and cAMP-dependent phosphorylation. J Biol Chem. 1999;274:16788–16795. doi: 10.1074/jbc.274.24.16788. [DOI] [PubMed] [Google Scholar]
- van Holde KE, Miller KI, Decker H. Hemocyanins and invertebrate evolution. J Biol Chem. 2001;276:15563–15566. doi: 10.1074/jbc.R100010200. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Johnson TJ, Myers AA, Kanost MR. Identification by subtractive suppression hybridization of bacteria-induced genes expressed in Manduca sexta fat body. Insect Biochem Mol Biol. 2003;33:541–559. doi: 10.1016/s0965-1748(03)00028-6. [DOI] [PubMed] [Google Scholar]