Abstract
Fermenting anaerobic cultures of Escherichia coli were observed by the nonintrusive technique of in vivo, whole-culture nuclear magnetic resonance. Fermentation balances were calculated for hexoses, pentoses, sugar alcohols, and sugar acids. Substrates more reduced than glucose yielded more of the highly reduced fermentation product ethanol, whereas more-oxidized substrates produced more of the less-reduced fermentation product acetate. These relationships were made more obvious by the introduction of ldhA mutations, which abolished lactate production, and delta frd mutations, which eliminated succinate. When grown anaerobically on sugar alcohols such as sorbitol, E. coli produced ethanol in excess of the amount calculated by the standard fermentation pathways. Reducing equivalents must be recycled from formate to account for this excess of ethanol. In mutants deficient in hydrogenase (hydB), ethanol production from sorbitol was greatly decreased, implying that hydrogen gas released from formate by the formate-hydrogen lyase system may be partially recycled, in the wild type, to increase the yield of the highly reduced fermentation product ethanol.
Full text
PDF
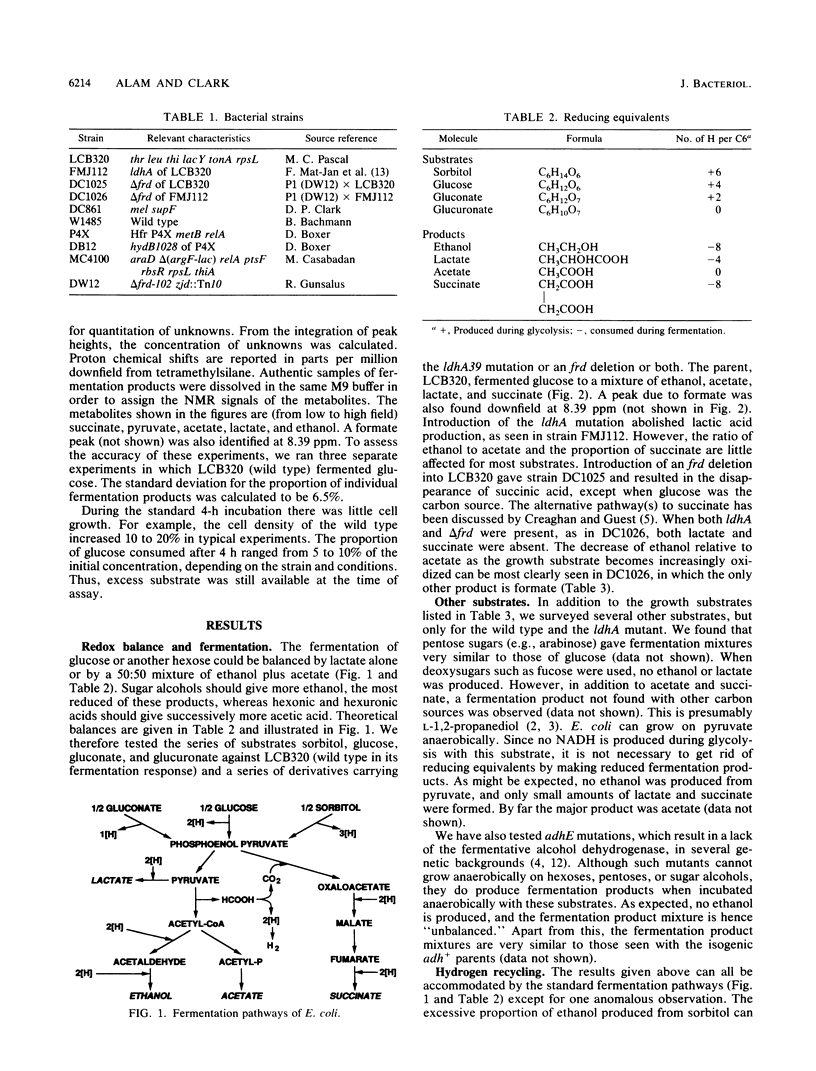

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACKWOOD A. C., LEDINGHAM G. A., NEISH A. C. Dissimilation of glucose at controlled pH values by pigmented and non-pigmented strains of Escherichia coli. J Bacteriol. 1956 Oct;72(4):497–499. doi: 10.1128/jb.72.4.497-499.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boronat A., Aguilar J. Metabolism of L-fucose and L-rhamnose in Escherichia coli: differences in induction of propanediol oxidoreductase. J Bacteriol. 1981 Jul;147(1):181–185. doi: 10.1128/jb.147.1.181-185.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. M., Lin E. C., Ros J., Aguilar J. Use of operon fusions to examine the regulation of the L-1,2-propanediol oxidoreductase gene of the fucose system in Escherichia coli K12. J Gen Microbiol. 1983 Nov;129(11):3355–3362. doi: 10.1099/00221287-129-11-3355. [DOI] [PubMed] [Google Scholar]
- Clark D. P., Cunningham P. R., Reams S. G., Mat-Jan F., Mohammedkhani R., Williams C. R. Mutants of Escherichia coli defective in acid fermentation. Appl Biochem Biotechnol. 1988 Apr;17:163–173. doi: 10.1007/BF02779155. [DOI] [PubMed] [Google Scholar]
- Creaghan I. T., Guest J. R. Succinate dehydrogenase-dependent nutritional requirement for succinate in mutants of Escherichia coli K12. J Gen Microbiol. 1978 Jul;107(1):1–13. doi: 10.1099/00221287-107-1-1. [DOI] [PubMed] [Google Scholar]
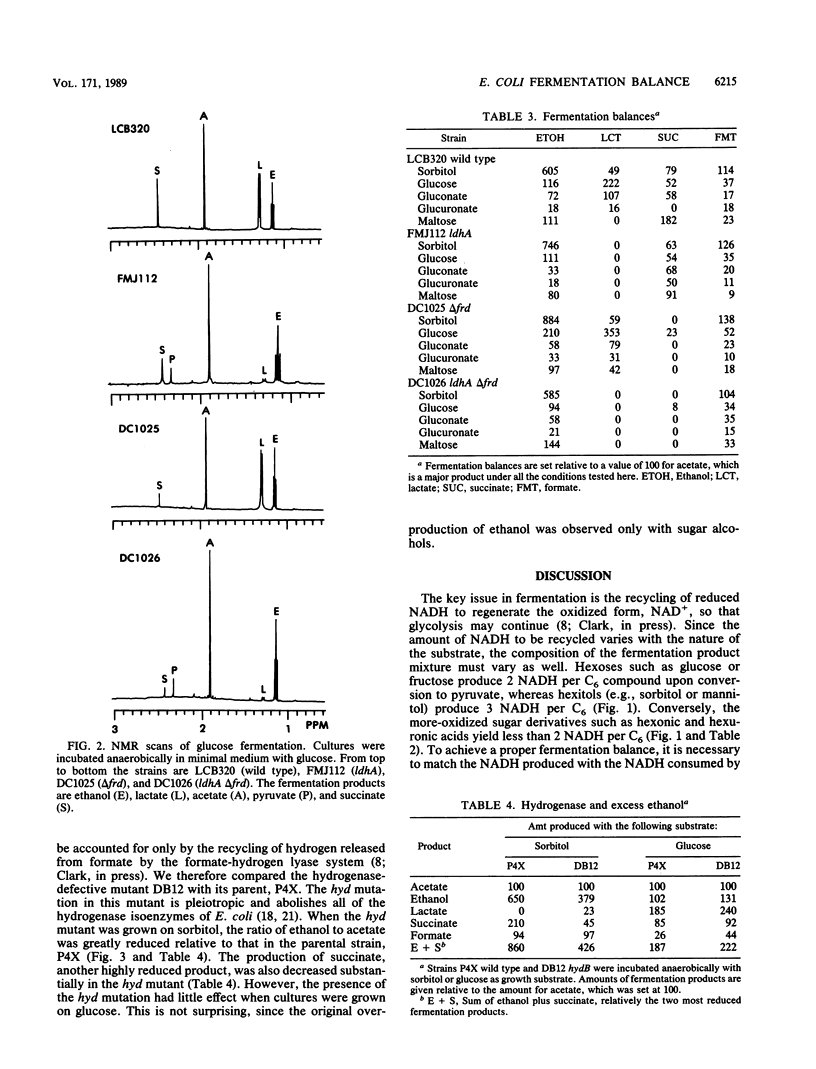
- Cunningham P. R., Clark D. P. The use of suicide substrates to select mutants of Escherichia coli lacking enzymes of alcohol fermentation. Mol Gen Genet. 1986 Dec;205(3):487–493. doi: 10.1007/BF00338087. [DOI] [PubMed] [Google Scholar]
- DAWES E. A., FOSTER S. M. The formation of ethanol in Escherichia coli. Biochim Biophys Acta. 1956 Nov;22(2):253–265. doi: 10.1016/0006-3002(56)90148-2. [DOI] [PubMed] [Google Scholar]
- Guest J. R. Anaerobic growth of Escherichia coli K12 with fumarate as terminal electron acceptor. Genetic studies with menaquinone and fluoroacetate-resistant mutants. J Gen Microbiol. 1979 Dec;115(2):259–271. doi: 10.1099/00221287-115-2-259. [DOI] [PubMed] [Google Scholar]
- Gupta S., Clark D. P. Escherichia coli derivatives lacking both alcohol dehydrogenase and phosphotransacetylase grow anaerobically by lactate fermentation. J Bacteriol. 1989 Jul;171(7):3650–3655. doi: 10.1128/jb.171.7.3650-3655.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingledew W. J., Poole R. K. The respiratory chains of Escherichia coli. Microbiol Rev. 1984 Sep;48(3):222–271. doi: 10.1128/mr.48.3.222-271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorowitz W., Clark D. Escherichia coli mutants with a temperature-sensitive alcohol dehydrogenase. J Bacteriol. 1982 Nov;152(2):935–938. doi: 10.1128/jb.152.2.935-938.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mat-Jan F., Alam K. Y., Clark D. P. Mutants of Escherichia coli deficient in the fermentative lactate dehydrogenase. J Bacteriol. 1989 Jan;171(1):342–348. doi: 10.1128/jb.171.1.342-348.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino T., Arata Y., Fujiwara S. Proton correlation nuclear magnetic resonance study of metabolic regulations and pyruvate transport in anaerobic Escherichia coli cells. Biochemistry. 1980 Aug 5;19(16):3684–3691. doi: 10.1021/bi00557a008. [DOI] [PubMed] [Google Scholar]
- Ogino T., Arata Y., Fujiwara S., Shoun H., Beppu T. Proton correlation nuclear magnetic resonance study of anaerobic metabolism of Escherichia coli. Biochemistry. 1978 Oct 31;17(22):4742–4745. doi: 10.1021/bi00615a022. [DOI] [PubMed] [Google Scholar]
- PAEGE L. M., GIBBS M. Anaerobic dissimilation of glucose-C14 by Escherichia coli. J Bacteriol. 1961 Jan;81:107–110. doi: 10.1128/jb.81.1.107-110.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
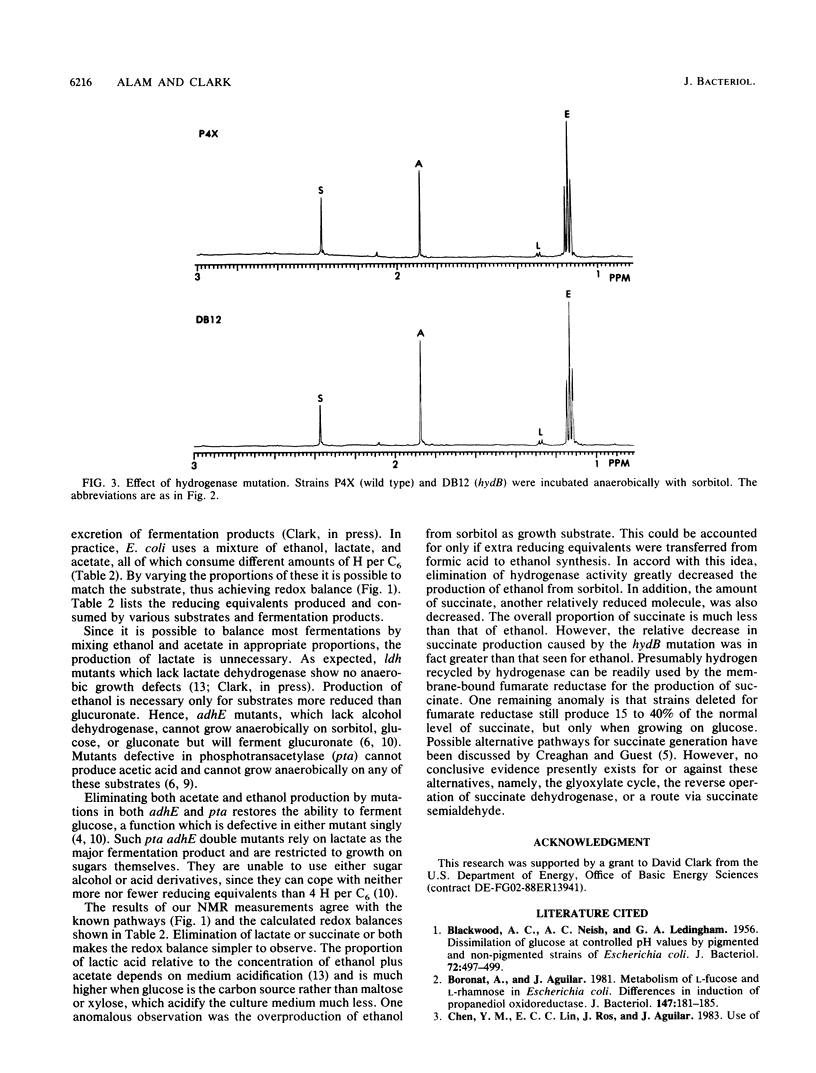
- Sawers R. G., Ballantine S. P., Boxer D. H. Differential expression of hydrogenase isoenzymes in Escherichia coli K-12: evidence for a third isoenzyme. J Bacteriol. 1985 Dec;164(3):1324–1331. doi: 10.1128/jb.164.3.1324-1331.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes J. L. FERMENTATION OF GLUCOSE BY SUSPENSIONS OF ESCHERICHIA COLI. J Bacteriol. 1949 Feb;57(2):147–158. doi: 10.1128/jb.57.2.147-158.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waugh R., Boxer D. H. Pleiotropic hydrogenase mutants of Escherichia coli K12: growth in the presence of nickel can restore hydrogenase activity. Biochimie. 1986 Jan;68(1):157–166. doi: 10.1016/s0300-9084(86)81080-x. [DOI] [PubMed] [Google Scholar]



