Abstract
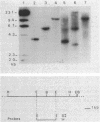
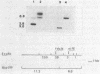
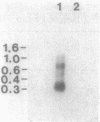
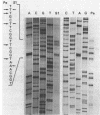
The structural gene (fdxN) encoding ferredoxin I (FdI) in the photosynthetic bacterium Rhodobacter capsulatus was isolated from a cosmid library by using an oligonucleotide probe corresponding to the N-terminal amino acid sequence of FdI. The nucleotide sequences of the gene and of the 3'- and 5'-flanking regions were determined. The gene fdxN codes for a polypeptide of 64 mino acids having a calculated molecular weight of 6,728. Amino acid sequencing of the N- and C-terminal ends of FdI allowed the determination of 86% of the primary structure and confirmed that FdI is the fdxN gene product. Sequence comparisons indicate that FdI shares common structural features with ferredoxins containing two [4Fe-4S] clusters, including eight conserved cysteines. Maximal homology was found with a ferredoxin from Rhodo-pseudomonas palustris. Northern (RNA) hybridization using a 158-base-pair DNA fragment internal to the fdxN coding region revealed the existence of two mRNA transcripts of approximately 330 and 750 nucleotides. Neither of those transcripts was present under nif-repressing growth conditions. The 5' end of the smaller transcript was mapped by S1 nuclease protection and primer extension experiments. On the basis of Southern hybridization experiments, by using probes homologous to fdxN, nifE, and a fragment complementing a nif point mutation, fdxN was localized inside a cluster of nif genes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. W., Forrest M. E., Cohen S. N., Beatty J. T. Structural and functional analysis of transcriptional control of the Rhodobacter capsulatus puf operon. J Bacteriol. 1989 Jan;171(1):473–482. doi: 10.1128/jb.171.1.473-482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avtges P., Kranz R. G., Haselkorn R. Isolation and organization of genes for nitrogen fixation in Rhodopseudomonas capsulata. Mol Gen Genet. 1985;201(3):363–369. doi: 10.1007/BF00331324. [DOI] [PubMed] [Google Scholar]
- Beynon J., Cannon M., Buchanan-Wollaston V., Cannon F. The nif promoters of Klebsiella pneumoniae have a characteristic primary structure. Cell. 1983 Sep;34(2):665–671. doi: 10.1016/0092-8674(83)90399-9. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Bruschi M., Guerlesquin F. Structure, function and evolution of bacterial ferredoxins. FEMS Microbiol Rev. 1988 Apr-Jun;4(2):155–175. doi: 10.1111/j.1574-6968.1988.tb02741.x. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Arnon D. I. Ferredoxins: chemistry and function in photosynthesis, nitrogen fixation, and fermentative metabolism. Adv Enzymol Relat Areas Mol Biol. 1970;33:119–176. doi: 10.1002/9780470122785.ch3. [DOI] [PubMed] [Google Scholar]
- Böhme H., Haselkorn R. Molecular cloning and nucleotide sequence analysis of the gene coding for heterocyst ferredoxin from the cyanobacterium Anabaena sp. strain PCC 7120. Mol Gen Genet. 1988 Oct;214(2):278–285. doi: 10.1007/BF00337722. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Ebeling S., Noti J. D., Hennecke H. Identification of a new Bradyrhizobium japonicum gene (frxA) encoding a ferredoxinlike protein. J Bacteriol. 1988 Apr;170(4):1999–2001. doi: 10.1128/jb.170.4.1999-2001.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eck R. V., Dayhoff M. O. Evolution of the structure of ferredoxin based on living relics of primitive amino Acid sequences. Science. 1966 Apr 15;152(3720):363–366. doi: 10.1126/science.152.3720.363. [DOI] [PubMed] [Google Scholar]
- Fitch W. M., Bruschi M. The evolution of prokaryotic ferredoxins--with a general method correcting for unobserved substitutions in less branched lineages. Mol Biol Evol. 1987 Jul;4(4):381–394. doi: 10.1093/oxfordjournals.molbev.a040452. [DOI] [PubMed] [Google Scholar]
- Fitch W. M. Evidence suggesting a partial, internal duplication in the ancestral gene for heme-containing globins. J Mol Biol. 1966 Mar;16(1):17–27. doi: 10.1016/s0022-2836(66)80259-0. [DOI] [PubMed] [Google Scholar]
- Fukuyama K., Nagahara Y., Tsukihara T., Katsube Y., Hase T., Matsubara H. Tertiary structure of Bacillus thermoproteolyticus [4Fe-4S] ferredoxin. Evolutionary implications for bacterial ferredoxins. J Mol Biol. 1988 Jan 5;199(1):183–193. doi: 10.1016/0022-2836(88)90388-9. [DOI] [PubMed] [Google Scholar]
- George D. G., Hunt L. T., Yeh L. S., Barker W. C. New perspectives on bacterial ferredoxin evolution. J Mol Evol. 1985;22(1):20–31. doi: 10.1007/BF02105801. [DOI] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Expression of a family of psbA genes encoding a photosystem II polypeptide in the cyanobacterium Anacystis nidulans R2. EMBO J. 1986 Nov;5(11):2789–2798. doi: 10.1002/j.1460-2075.1986.tb04569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase T., Matsubara H., Evans M. C. Amino acid sequence of chromatium vinosum ferredoxin: revisions. J Biochem. 1977 Jun;81(6):1745–1749. doi: 10.1093/oxfordjournals.jbchem.a131635. [DOI] [PubMed] [Google Scholar]
- Hase T., Wakabayashi S., Matsubara H., Evans M. C., Jennings J. V. Amino acid sequence of a ferredoxin from Chlorobium thiosulfatophilum strain Tassajara, a photosynthetic green sulfur bacterium. J Biochem. 1978 May;83(5):1321–1325. doi: 10.1093/oxfordjournals.jbchem.a132039. [DOI] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Joerger R. D., Bishop P. E. Nucleotide sequence and genetic analysis of the nifB-nifQ region from Azotobacter vinelandii. J Bacteriol. 1988 Apr;170(4):1475–1487. doi: 10.1128/jb.170.4.1475-1487.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipp W., Masepohl B., Pühler A. Identification and mapping of nitrogen fixation genes of Rhodobacter capsulatus: duplication of a nifA-nifB region. J Bacteriol. 1988 Feb;170(2):693–699. doi: 10.1128/jb.170.2.693-699.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klipp W., Reiländer H., Schlüter A., Krey R., Pühler A. The Rhizobium meliloti fdxN gene encoding a ferredoxin-like protein is necessary for nitrogen fixation and is cotranscribed with nifA and nifB. Mol Gen Genet. 1989 Apr;216(2-3):293–302. doi: 10.1007/BF00334368. [DOI] [PubMed] [Google Scholar]
- Leclerc M., Colbeau A., Cauvin B., Vignais P. M. Cloning and sequencing of the genes encoding the large and the small subunits of the H2 uptake hydrogenase (hup) of Rhodobacter capsulatus. Mol Gen Genet. 1988 Sep;214(1):97–107. doi: 10.1007/BF00340186. [DOI] [PubMed] [Google Scholar]
- Marrs B. Genetic recombination in Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1974 Mar;71(3):971–973. doi: 10.1073/pnas.71.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masepohl B., Klipp W., Pühler A. Genetic characterization and sequence analysis of the duplicated nifA/nifB gene region of Rhodobacter capsulatus. Mol Gen Genet. 1988 Apr;212(1):27–37. doi: 10.1007/BF00322441. [DOI] [PubMed] [Google Scholar]
- Matsubara H., Inoue K., Hase T., Hiura H., Kakuno T., Yamashita J., Horio T. Structure of the extracellular ferredoxin from Rhodospirillum rubrum: close similarity to clostridial ferredoxins. J Biochem. 1983 May;93(5):1385–1390. doi: 10.1093/oxfordjournals.jbchem.a134273. [DOI] [PubMed] [Google Scholar]
- Matsubara H., Sasaki R. M., Tsuchiya D. K., Evans M. C. The amino acid sequence of Chromatium ferredoxin. J Biol Chem. 1970 Apr 25;245(8):2121–2131. [PubMed] [Google Scholar]
- Minami Y., Wakabayashi S., Yamada F., Wada K., Zumft W. G., Matsubara H. Ferredoxins from the photosynthetic purple non-sulfur bacterium Rhodopseudomonas palustris. Isolation and amino acid sequence of ferredoxin I. J Biochem. 1984 Sep;96(3):585–592. doi: 10.1093/oxfordjournals.jbchem.a134873. [DOI] [PubMed] [Google Scholar]
- Moreno-Vivian C., Hennecke S., Pühler A., Klipp W. Open reading frame 5 (ORF5), encoding a ferredoxinlike protein, and nifQ are cotranscribed with nifE, nifN, nifX, and ORF4 in Rhodobacter capsulatus. J Bacteriol. 1989 May;171(5):2591–2598. doi: 10.1128/jb.171.5.2591-2598.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Vivian C., Schmehl M., Masepohl B., Arnold W., Klipp W. DNA sequence and genetic analysis of the Rhodobacter capsulatus nifENX gene region: homology between NifX and NifB suggests involvement of NifX in processing of the iron-molybdenum cofactor. Mol Gen Genet. 1989 Apr;216(2-3):353–363. doi: 10.1007/BF00334376. [DOI] [PubMed] [Google Scholar]
- Morgan T. V., Lundell D. J., Burgess B. K. Azotobacter vinelandii ferredoxin I: cloning, sequencing, and mutant analysis. J Biol Chem. 1988 Jan 25;263(3):1370–1375. [PubMed] [Google Scholar]
- Moulis J. M., Meyer J. Characterization of the selenium-substituted 2 [4Fe-4Se] ferredoxin from Clostridium pasteurianum. Biochemistry. 1982 Sep 14;21(19):4762–4771. doi: 10.1021/bi00262a037. [DOI] [PubMed] [Google Scholar]
- Mulligan M. E., Buikema W. J., Haselkorn R. Bacterial-type ferredoxin genes in the nitrogen fixation regions of the cyanobacterium Anabaena sp. strain PCC 7120 and Rhizobium meliloti. J Bacteriol. 1988 Sep;170(9):4406–4410. doi: 10.1128/jb.170.9.4406-4410.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okawara N., Ogata M., Yagi T., Wakabayashi S., Matsubara H. Amino acid sequence of ferredoxin I from Desulfovibrio vulgaris Miyazaki. J Biochem. 1988 Aug;104(2):196–199. doi: 10.1093/oxfordjournals.jbchem.a122441. [DOI] [PubMed] [Google Scholar]
- Pollock D., Bauer C. E., Scolnik P. A. Transcription of the Rhodobacter capsulatus nifHDK operon is modulated by the nitrogen source. Construction of plasmid expression vectors based on the nifHDK promoter. Gene. 1988 May 30;65(2):269–275. doi: 10.1016/0378-1119(88)90463-5. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L. M. Characterization of translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2971–2996. doi: 10.1093/nar/10.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers D. J., Keim J. M., Paul P. S., Lyoo Y. S., Merion M., Benbow R. M. High-resolution chromatography of nucleic acids on the Gen-Pak FAX column. J Chromatogr. 1988 Jul 1;444:47–65. doi: 10.1016/s0021-9673(01)94008-7. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Haniu M., Yasunobu K. T., Evans M. C., Rao K. K. Amino acid sequence of ferredoxin from a photosynthetic green bacterium, Chlorobium limicola. Biochemistry. 1974 Jul 2;13(14):2953–2959. doi: 10.1021/bi00711a026. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Haniu M., Yasunobu K. T., Evans M. C., Rao K. K. The amino acid sequence of ferredoxin II from Chlorobium limicola, a photosynthetic green bacterium. Biochemistry. 1975 May 6;14(9):1938–1943. doi: 10.1021/bi00680a021. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Nakashima T., Benson A., Mower H., Tasunobu K. T. The amino acid sequence of Clostridium pasteurianum ferredoxin. Biochemistry. 1966 May;5(5):1666–1681. doi: 10.1021/bi00869a032. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yoch D. C., Arnon D. I. Comparison of two ferredoxins from Rhodospirillum rubrum as electron carriers for the native nitrogenase. J Bacteriol. 1975 Feb;121(2):743–745. doi: 10.1128/jb.121.2.743-745.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoch D. C., Arnon D. I., Sweeney W. V. Characterization of two soluble ferredoxins as distinct from bound iron-sulfur proteins in the photosynthetic bacterium Rhodospirillum rubrum. J Biol Chem. 1975 Nov 10;250(21):8330–8336. [PubMed] [Google Scholar]