Abstract
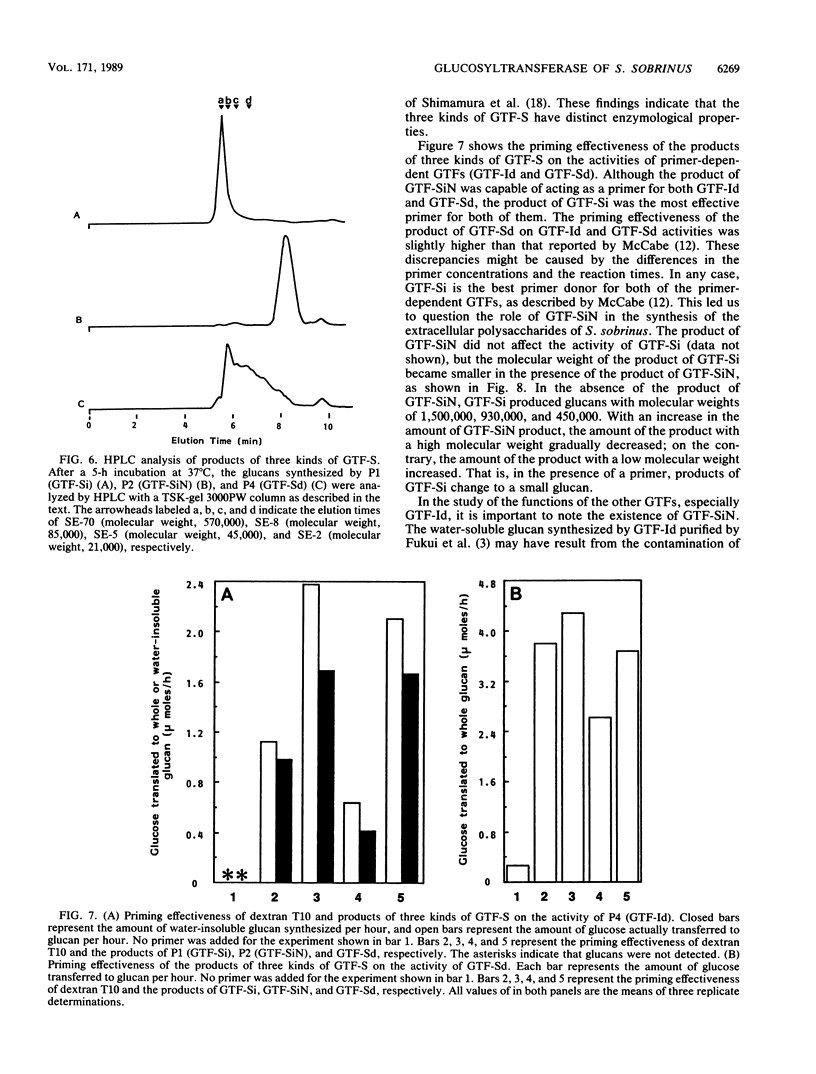
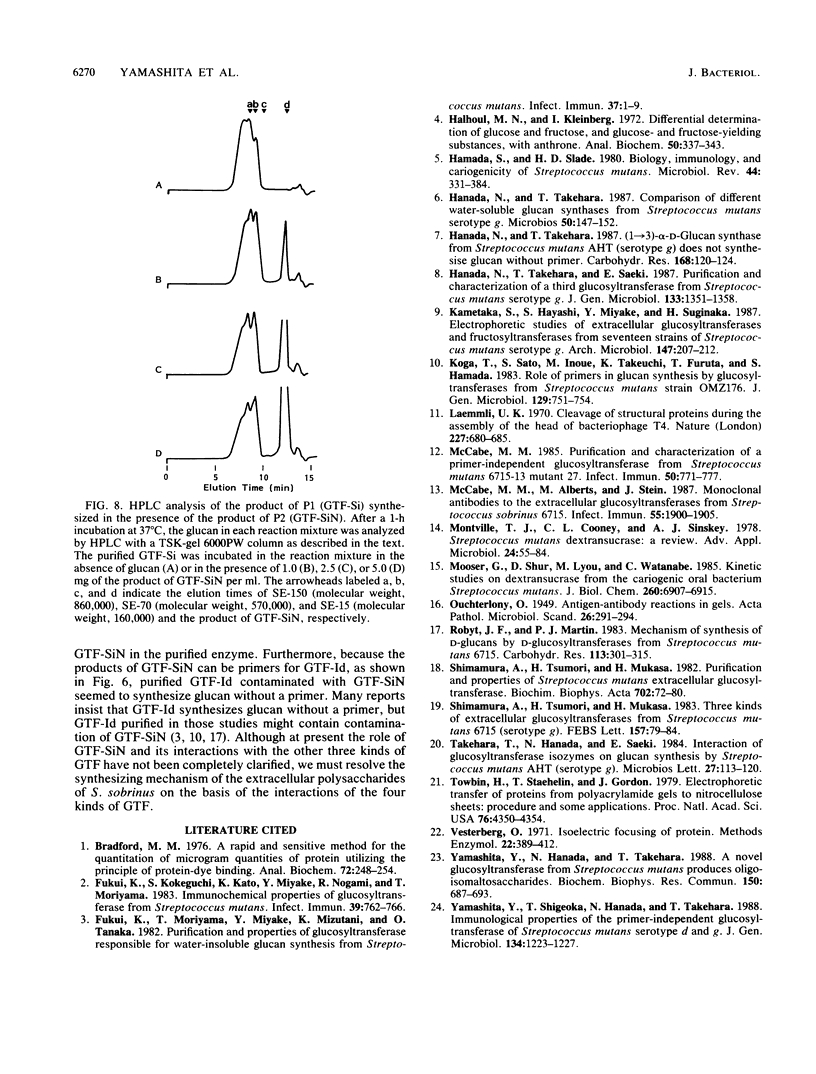
Recently, we found a novel primer-independent, water-soluble glucan synthase as a fourth glucosyltransferase (GTF) in a culture supernatant of strain AHT-k of Streptococcus sobrinus (Y. Yamashita, N. Hanada, and T. Takehara, Biochem. Biophys. Res. Commun. 150:687-693, 1988). In the present study, four kinds of purified GTFs, including the novel GTF, were prepared. They were composed of two primer-dependent GTFs and two primer-independent GTFs. Of the primer-dependent GTFs, one was a water-insoluble glucan synthase and the other was a water-soluble glucan synthase; both of the primer-independent GTFs were water-soluble glucan synthases (GTF-Sis). Using antisera against four purified GTFs, we concluded that the immunological properties of each were completely different from those of the others. Additionally, it was shown that the novel GTF-Si, which was previously shown to have a molecular weight of 137,000, was proteolytically degraded and could be isolated at a molecular weight of 152,000 and that Streptococcus cricetus secreted an enzyme that immunologically cross-reacted with GTF-Si. While the product of the novel GTF-Si was not an effective primer for both of the primer-dependent enzymes (water-soluble and -insoluble glucan synthases), the product of the enzyme affected the molecular size of the products of the other GTF-Sis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Fukui K., Kokeguchi S., Kato K., Miyake Y., Nogami R., Moriyama T. Immunochemical properties of glucosyltransferases from Streptococcus mutans. Infect Immun. 1983 Feb;39(2):762–766. doi: 10.1128/iai.39.2.762-766.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui K., Moriyama T., Miyake Y., Mizutani K., Tanaka O. Purification and properties of glucosyltransferase responsible for water-insoluble glucan synthesis from Streptococcus mutans. Infect Immun. 1982 Jul;37(1):1–9. doi: 10.1128/iai.37.1.1-9.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halhoul M. N., Kleinberg I. Differential determination of glucose and fructose, and glucose- and fructose-yielding substances with anthrone. Anal Biochem. 1972 Dec;50(2):337–343. doi: 10.1016/0003-2697(72)90042-5. [DOI] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada N., Takehara T. (1----3)-alpha-D-glucan synthase from Streptococcus mutans AHT (serotype g) does not synthesise glucan without primer. Carbohydr Res. 1987 Oct 15;168(1):120–124. doi: 10.1016/0008-6215(87)80013-7. [DOI] [PubMed] [Google Scholar]
- Hanada N., Takehara T. Comparison of different water-soluble glucan synthases from Streptococcus mutans serotype g. Microbios. 1987;50(204-205):147–152. [PubMed] [Google Scholar]
- Hanada N., Takehara T., Saeki E. Purification and characterization of a third glucosyltransferase from Streptococcus mutans serotype g. J Gen Microbiol. 1987 May;133(5):1351–1358. doi: 10.1099/00221287-133-5-1351. [DOI] [PubMed] [Google Scholar]
- Kametaka S., Hayashi S., Miyake Y., Suginaka H. Electrophoretic studies of extracellular glucosyltransferases and fructosyltransferases from seventeen strains of Streptococcus mutans. Arch Microbiol. 1987 Apr;147(3):207–212. doi: 10.1007/BF00463476. [DOI] [PubMed] [Google Scholar]
- Koga T., Sato S., Inoue M., Takeuchi K., Furuta T., Hamada S. Role of primers in glucan synthesis by glucosyltransferases from Streptococcus mutans strain OMZ176. J Gen Microbiol. 1983 Mar;129(3):751–754. doi: 10.1099/00221287-129-3-751. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McCabe M. M., Alberts M., Stein J. Monoclonal antibodies to the extracellular glucosyltransferases from Streptococcus sobrinus 6715. Infect Immun. 1987 Aug;55(8):1900–1905. doi: 10.1128/iai.55.8.1900-1905.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe M. M. Purification and characterization of a primer-independent glucosyltransferase from Streptococcus mutans 6715-13 mutant 27. Infect Immun. 1985 Dec;50(3):771–777. doi: 10.1128/iai.50.3.771-777.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montville T. J., Cooney C. L., Sinskey A. J. Streptococcus mutans dextransucrase: a review. Adv Appl Microbiol. 1978;24:55–84. doi: 10.1016/s0065-2164(08)70636-1. [DOI] [PubMed] [Google Scholar]
- Mooser G., Shur D., Lyou M., Watanabe C. Kinetic studies on dextransucrase from the cariogenic oral bacterium Streptococcus mutans. J Biol Chem. 1985 Jun 10;260(11):6907–6915. [PubMed] [Google Scholar]
- Robyt J. F., Martin P. J. Mechanism of synthesis of D-glucans by D-glucosyltransferases from Streptococcus mutans 6715. Carbohydr Res. 1983 Mar 1;113(2):301–315. doi: 10.1016/0008-6215(83)88245-7. [DOI] [PubMed] [Google Scholar]
- Shimamura A., Tsumori H., Mukasa H. Purification and properties of Streptococcus mutans extracellular glucosyltransferase. Biochim Biophys Acta. 1982 Mar 18;702(1):72–80. doi: 10.1016/0167-4838(82)90028-0. [DOI] [PubMed] [Google Scholar]
- Shimamura A., Tsumori H., Mukasa H. Three kinds of extracellular glucosyltransferases from Streptococcus mutans 6715 (serotype g). FEBS Lett. 1983 Jun 27;157(1):79–84. doi: 10.1016/0014-5793(83)81120-x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita Y., Hanada N., Takehara T. A novel glucosyltransferase from Streptococcus mutans produces oligo-isomaltosaccharides. Biochem Biophys Res Commun. 1988 Jan 29;150(2):687–693. doi: 10.1016/0006-291x(88)90446-9. [DOI] [PubMed] [Google Scholar]
- Yamashita Y., Shigeoka T., Hanada N., Takehara T. Immunological properties of the primer-independent glucosyltransferase of Streptococcus mutans serotypes d and g. J Gen Microbiol. 1988 May;134(5):1223–1227. doi: 10.1099/00221287-134-5-1223. [DOI] [PubMed] [Google Scholar]