Abstract
Laccase from the ascomycete Neurospora crassa is an inducible secretory enzyme. Production of this enzyme is repressed in vegetative cultures but can be induced by treatment with low concentrations of cycloheximide. Isolation and characterization of a derepressed mutant, the lah-1 mutant, that is capable of producing laccase in vegetative cultures without induction by cycloheximide are described. The lah-1 mutation is mapped between nit-2 and leu-3 on linkage group I, and it behaved as a recessive mutation in a forced heterokaryon. No differences were detected biochemically or immunologically between the laccase protein produced by the lah-1 mutant in the absence of cycloheximide and that induced with cycloheximide in the wild-type strain. This suggests that both laccases (66 kilodaltons) are products of the same structural gene. Relative amounts of laccase in the culture filtrate of the lah-1 mutant were much higher than those induced with cycloheximide in the wild-type strain, demonstrating high efficiency of the lah-1 mutant in production and secretion of laccase. The time course of laccase production by the lah-1 mutant revealed that expression of 66-kilodalton laccase was repressed in conidia and derepressed during vegetative mycelial growth. This suggests that a multiple regulatory mechanism is involved in the production and/or maturation of Neurospora laccase. The lah-1 mutant may be useful for identifying genes that regulate expression of the laccase gene in N. crassa.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boucherie H., Dupont C. H., Bernet J. Polypeptide synthesis during protoplasmic incompatibility in the fungus Podospora anserina. Biochim Biophys Acta. 1981 Mar 26;653(1):18–26. doi: 10.1016/0005-2787(81)90100-3. [DOI] [PubMed] [Google Scholar]
- Clutterbuck A. J. Absence of laccase from yellow-spored mutants of Aspergillus nidulans. J Gen Microbiol. 1972 May;70(3):423–435. doi: 10.1099/00221287-70-3-423. [DOI] [PubMed] [Google Scholar]
- Froehner S. C., Eriksson K. E. Induction of Neurospora crassa laccase with protein synthesis inhibitors. J Bacteriol. 1974 Oct;120(1):450–457. doi: 10.1128/jb.120.1.450-457.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehner S. C., Eriksson K. E. Purification and properties of Neurospora crassa laccase. J Bacteriol. 1974 Oct;120(1):458–465. doi: 10.1128/jb.120.1.458-465.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germann U. A., Lerch K. Isolation and partial nucleotide sequence of the laccase gene from Neurospora crassa: amino acid sequence homology of the protein to human ceruloplasmin. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8854–8858. doi: 10.1073/pnas.83.23.8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
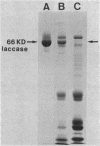
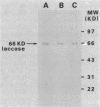
- Germann U. A., Müller G., Hunziker P. E., Lerch K. Characterization of two allelic forms of Neurospora crassa laccase. Amino- and carboxyl-terminal processing of a precursor. J Biol Chem. 1988 Jan 15;263(2):885–896. [PubMed] [Google Scholar]
- Gratzner H., Sheehan D. N. Neurospora mutant exhibiting hyperproduction of amylase and invertase. J Bacteriol. 1969 Feb;97(2):544–549. doi: 10.1128/jb.97.2.544-549.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H., Harvey R. C., Callen D. F., de Serres F. J. Mutagenesis at the ad-3A and ad-3B loci in haploid UV-sensitive strains of Neurospora crassa. V. Comparison of dose--response curves of single- and double-mutant strains with wild-type. Mutat Res. 1981 Nov;84(1):49–71. doi: 10.1016/0027-5107(81)90049-x. [DOI] [PubMed] [Google Scholar]
- LEVY H. B., SOBER H. A. A simple chromatographic method for preparation of gamma globulin. Proc Soc Exp Biol Med. 1960 Jan;103:250–252. doi: 10.3181/00379727-103-25476. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Law D. J., Timberlake W. E. Developmental regulation of laccase levels in Aspergillus nidulans. J Bacteriol. 1980 Nov;144(2):509–517. doi: 10.1128/jb.144.2.509-517.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. B., Free S. J. Isolation and characterization of Neurospora mutants affected in invertase synthesis. Genetics. 1984 Apr;106(4):591–599. doi: 10.1093/genetics/106.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch K., Deinum J., Reinhammar B. The state of copper in Neurospora laccase. Biochim Biophys Acta. 1978 May 24;534(1):7–14. doi: 10.1016/0005-2795(78)90470-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]