Abstract
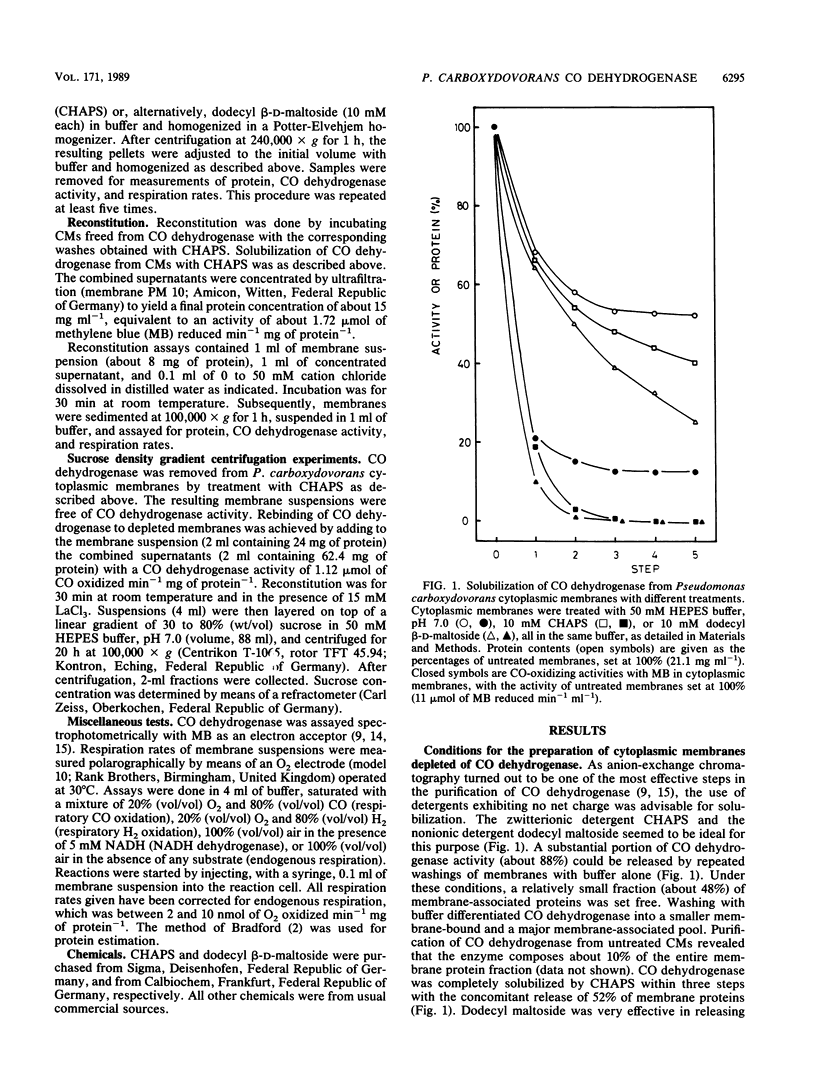
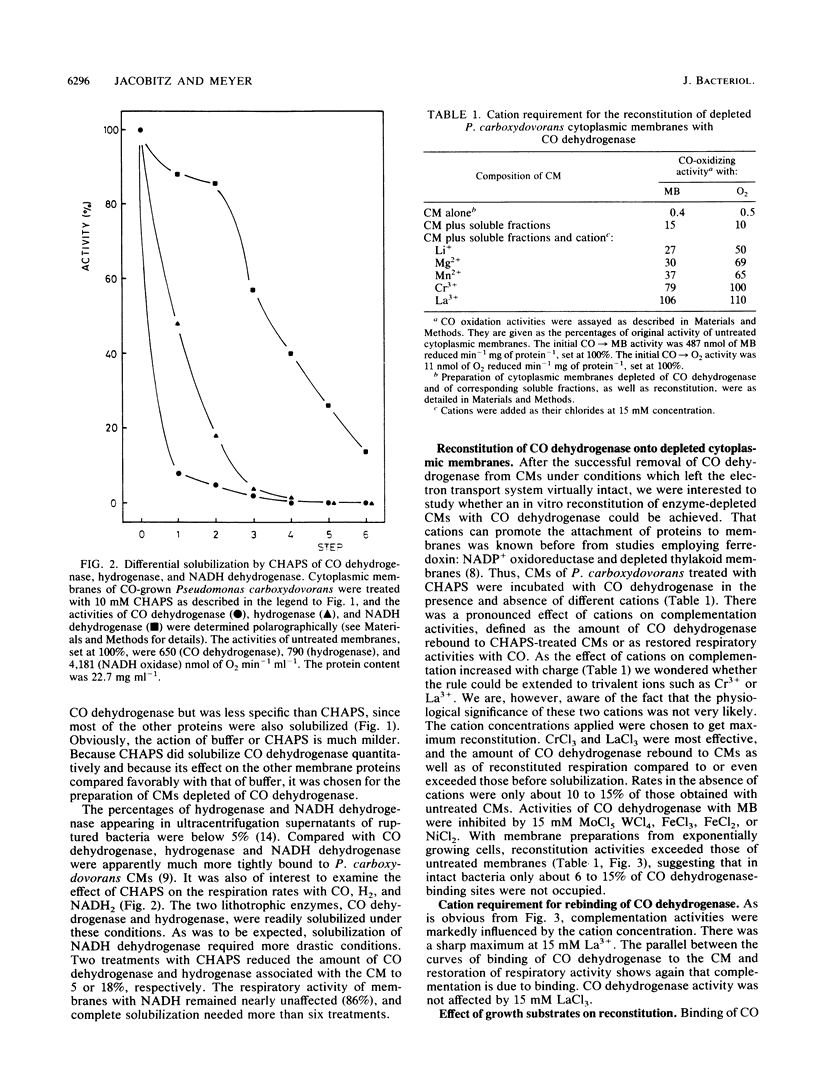
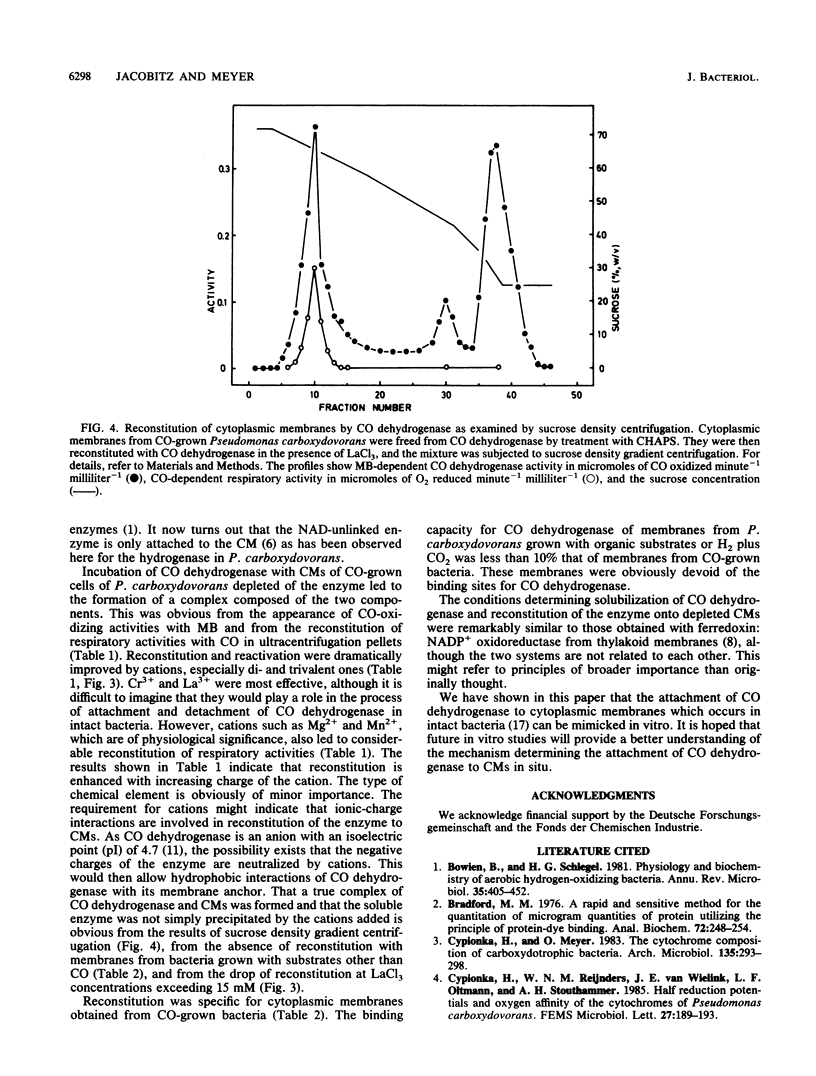
In Pseudomonas carboxydovorans, CO dehydrogenase and hydrogenase were found in association with the cytoplasmic membrane in a weakly bound and a tightly bound pool. The pools could be experimentally distinguished on the basis of resistance to removal by washes in low-ionic-strength buffer. The tightly bound pool of the enzymes could be differentially solubilized under conditions leaving the electron transport system intact and with the nondenaturing zwitterionic detergent 3-(3-cholamidopropyl) dimethylammonio 1-propane-sulfonic acid (CHAPS) and the nonionic detergent dodecyl beta-D-maltoside. In vitro reconstitution of depleted membranes with the corresponding supernatants containing CO dehydrogenase led to binding of the enzyme and to reactivation of respiratory activities with CO. The reconstitution reaction required cations with effectiveness which increased with increasing ionic charge: monovalent (Li+), divalent (Mg2+, Mn2+), or trivalent (Cr3+, La3+). Reconstitution of depleted membranes with CO dehydrogenase was specific for CO-grown bacteria. Cytoplasmic membranes from H2- or heterotrophically grown Pseudomonas carboxydovorans had no affinity for CO dehydrogenase at all, indicating the absence of the physiological electron acceptor of the enzyme, which presumably is cytochrome b561, or another membrane anchor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowien B., Schlegel H. G. Physiology and biochemistry of aerobic hydrogen-oxidizing bacteria. Annu Rev Microbiol. 1981;35:405–452. doi: 10.1146/annurev.mi.35.100181.002201. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Matthijs H. C., Coughlan S. J., Hind G. Removal of ferredoxin:NADP+ oxidoreductase from thylakoid membranes, rebinding to depleted membranes, and identification of the binding site. J Biol Chem. 1986 Sep 15;261(26):12154–12158. [PubMed] [Google Scholar]
- Meyer O. Chemical and spectral properties of carbon monoxide: methylene blue oxidoreductase. The molybdenum-containing iron-sulfur flavoprotein from Pseudomonas carboxydovorans. J Biol Chem. 1982 Feb 10;257(3):1333–1341. [PubMed] [Google Scholar]
- Meyer O., Schlegel H. G. Biology of aerobic carbon monoxide-oxidizing bacteria. Annu Rev Microbiol. 1983;37:277–310. doi: 10.1146/annurev.mi.37.100183.001425. [DOI] [PubMed] [Google Scholar]
- Meyer O., Schlegel H. G. Carbon monoxide:methylene blue oxidoreductase from Pseudomonas carboxydovorans. J Bacteriol. 1980 Jan;141(1):74–80. doi: 10.1128/jb.141.1.74-80.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer O., Schlegel H. G. Oxidation of carbon monoxide in cell extracts of Pseudomonas carboxydovorans. J Bacteriol. 1979 Feb;137(2):811–817. doi: 10.1128/jb.137.2.811-817.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde M., Mayer F., Meyer O. Immunocytochemical localization of carbon monoxide oxidase in Pseudomonas carboxydovorans. The enzyme is attached to the inner aspect of the cytoplasmic membrane. J Biol Chem. 1984 Dec 10;259(23):14788–14792. [PubMed] [Google Scholar]



