Abstract
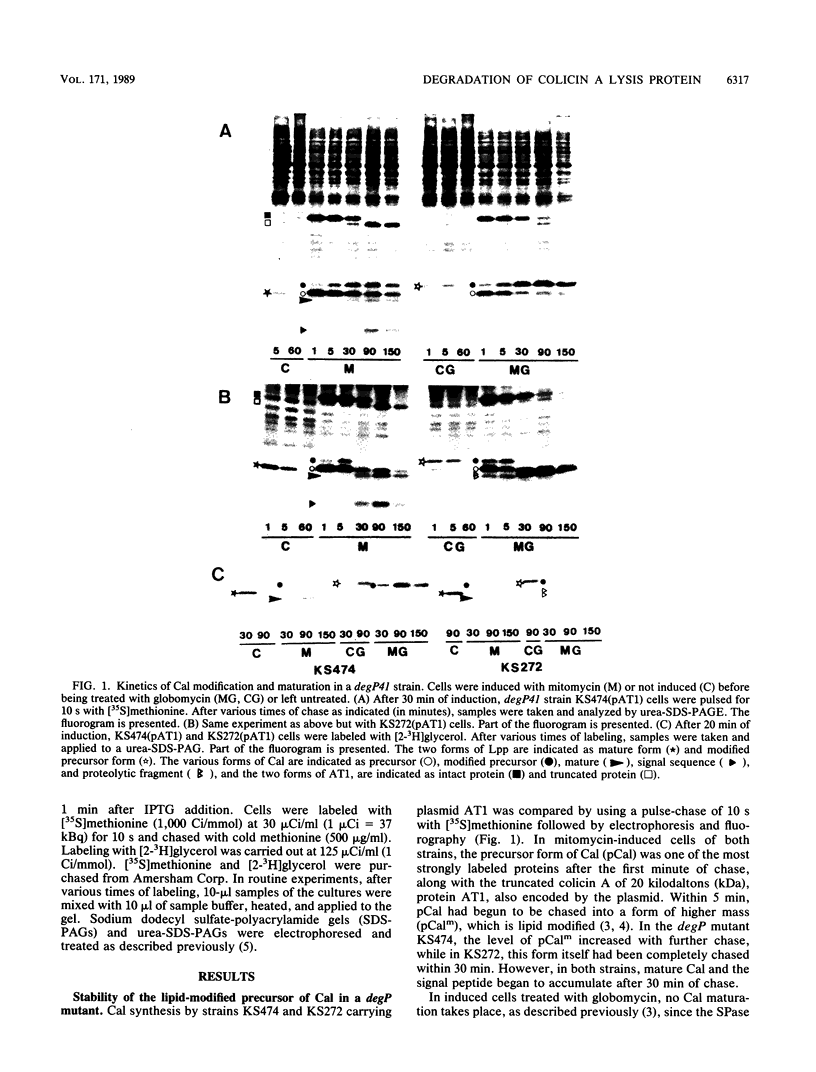
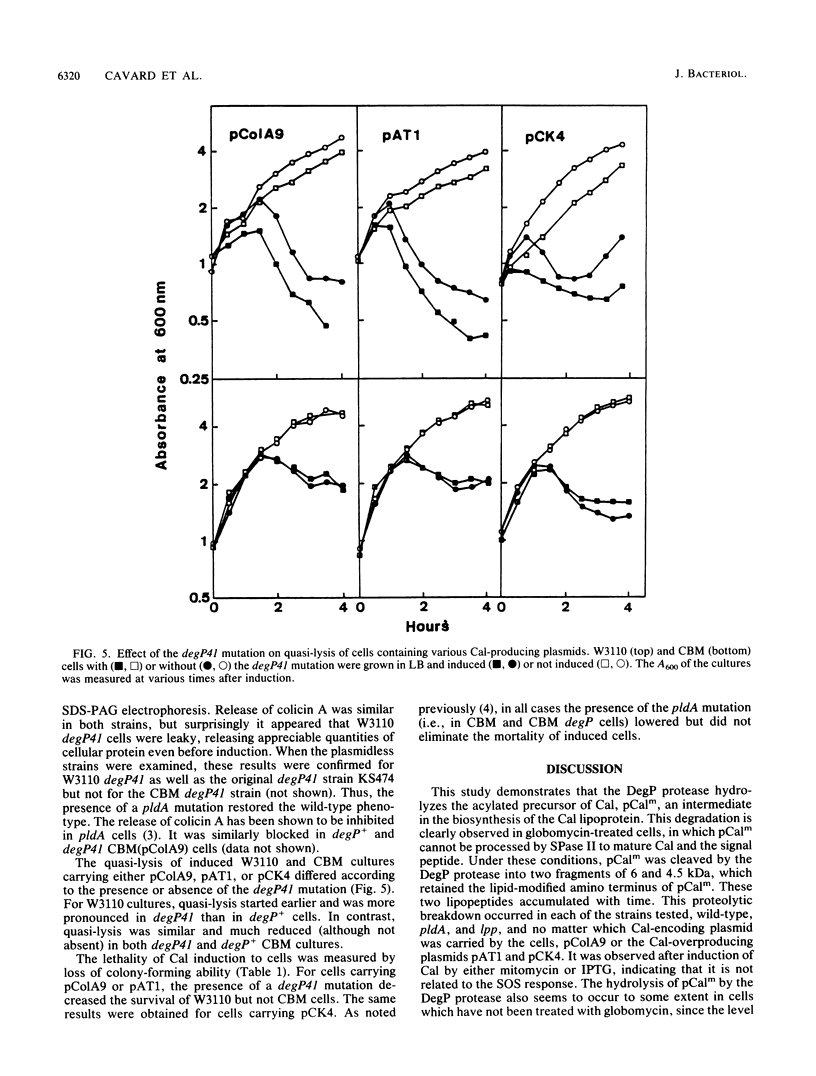
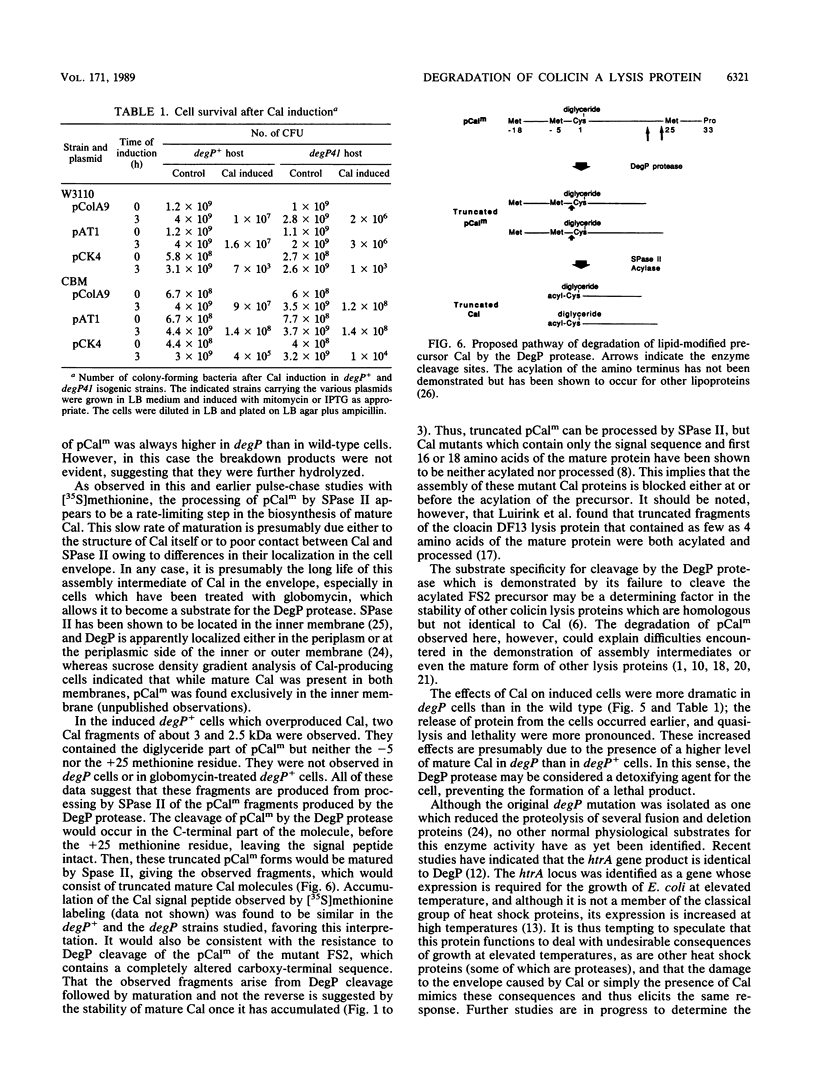
The acylated precursor form of the colicin A lysis protein (pCalm) is specifically cleaved by the DegP protease into two acylated fragments of 6 and 4.5 kilodaltons (kDa). This cleavage was observed after globomycin treatment, which inhibits the processing of pCalm into mature colicin A lysis protein (Cal) and the signal peptide. The cleavage took place in lpp, pldA, and wild-type strans carrying plasmids which express the lysis protein following SOS induction and also in cells containing a plasmid which expresses it under the control of the tac promoter. Furthermore, the DegP protease was responsible for the production of two acylated Cal fragments of 3 and 2.5 kDa in cells carrying plasmids which overproduce the Cal protein, without treatment with globomycin. DegP could also cleave the acylated precursor form of a mutant Cal protein containing a substitution in he amino-terminal portion of the protein, but not that of a mutant Cal containing a frameshift mutation in its carboxyl-terminal end. The functions of Cal in causing protein release, quasi-lysis, and lethality were increased in degP41 cells, suggesting that mature Cal was produced in higher amounts in the mutant than in the wild type. These effects were limited in cells deficient in phospholipase A. Interactions between the DegP protease and phospholipase A were suggested by the characteristics of degP pldA double mutants.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altieri M., Suit J. L., Fan M. L., Luria S. E. Expression of the cloned ColE1 kil gene in normal and Kilr Escherichia coli. J Bacteriol. 1986 Nov;168(2):648–654. doi: 10.1128/jb.168.2.648-654.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baty D., Lloubès R., Geli V., Lazdunski C., Howard S. P. Extracellular release of colicin A is non-specific. EMBO J. 1987 Aug;6(8):2463–2468. doi: 10.1002/j.1460-2075.1987.tb02526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavard D., Baty D., Howard S. P., Verheij H. M., Lazdunski C. Lipoprotein nature of the colicin A lysis protein: effect of amino acid substitutions at the site of modification and processing. J Bacteriol. 1987 May;169(5):2187–2194. doi: 10.1128/jb.169.5.2187-2194.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavard D., Howard S. P., Lloubes R., Lazdunski C. High-level expression of the colicin A lysis protein. Mol Gen Genet. 1989 Jun;217(2-3):511–519. doi: 10.1007/BF02464925. [DOI] [PubMed] [Google Scholar]
- Cavard D., Lloubès R., Morlon J., Chartier M., Lazdunski C. Lysis protein encoded by plasmid ColA-CA31. Gene sequence and export. Mol Gen Genet. 1985;199(1):95–100. doi: 10.1007/BF00327516. [DOI] [PubMed] [Google Scholar]
- De Graaf F. K., Oudega B. Production and release of cloacin DF13 and related colicins. Curr Top Microbiol Immunol. 1986;125:183–205. doi: 10.1007/978-3-642-71251-7_11. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Suzuki H., Nishimura Y., Yasuda S. On the process of cellular division in Escherichia coli: a mutant of E. coli lacking a murein-lipoprotein. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1417–1420. doi: 10.1073/pnas.74.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard S. P., Cavard D., Lazdunski C. Amino acid sequence and length requirements for assembly and function of the colicin A lysis protein. J Bacteriol. 1989 Jan;171(1):410–418. doi: 10.1128/jb.171.1.410-418.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inukai M., Takeuchi M., Shimizu K., Arai M. Mechanism of action of globomycin. J Antibiot (Tokyo) 1978 Nov;31(11):1203–1205. doi: 10.7164/antibiotics.31.1203. [DOI] [PubMed] [Google Scholar]
- Jakes K. S., Zinder N. D. Plasmid ColE3 specifies a lysis protein. J Bacteriol. 1984 Feb;157(2):582–590. doi: 10.1128/jb.157.2.582-590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knibiehler M., Howard S. P., Baty D., Geli V., Lloubès R., Sauve P., Lazdunski C. Isolation and molecular and functional properties of the amino-terminal domain of colicin A. Eur J Biochem. 1989 Apr 15;181(1):109–113. doi: 10.1111/j.1432-1033.1989.tb14700.x. [DOI] [PubMed] [Google Scholar]
- Lipinska B., Fayet O., Baird L., Georgopoulos C. Identification, characterization, and mapping of the Escherichia coli htrA gene, whose product is essential for bacterial growth only at elevated temperatures. J Bacteriol. 1989 Mar;171(3):1574–1584. doi: 10.1128/jb.171.3.1574-1584.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinska B., Sharma S., Georgopoulos C. Sequence analysis and regulation of the htrA gene of Escherichia coli: a sigma 32-independent mechanism of heat-inducible transcription. Nucleic Acids Res. 1988 Nov 11;16(21):10053–10067. doi: 10.1093/nar/16.21.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Lloubes R., Baty D., Lazdunski C. The promoters of the genes for colicin production, release and immunity in the ColA plasmid: effects of convergent transcription and Lex A protein. Nucleic Acids Res. 1986 Mar 25;14(6):2621–2636. doi: 10.1093/nar/14.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloubès R., Baty D., Lazdunski C. Transcriptional terminators in the caa-cal operon and cai gene. Nucleic Acids Res. 1988 May 11;16(9):3739–3749. doi: 10.1093/nar/16.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luirink J., Clark D. M., Ras J., Verschoor E. J., Stegehuis F., de Graaf F. K., Oudega B. pCloDF13-encoded bacteriocin release proteins with shortened carboxyl-terminal segments are lipid modified and processed and function in release of cloacin DF13 and apparent host cell lysis. J Bacteriol. 1989 May;171(5):2673–2679. doi: 10.1128/jb.171.5.2673-2679.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luirink J., Watanabe T., Wu H. C., Stegehuis F., de Graaf F. K., Oudega B. Modification, processing, and subcellular localization in Escherichia coli of the pCloDF13-encoded bacteriocin release protein fused to the mature portion of beta-lactamase. J Bacteriol. 1987 May;169(5):2245–2250. doi: 10.1128/jb.169.5.2245-2250.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Schwartz M. Colicin E2 release: lysis, leakage or secretion? Possible role of a phospholipase. EMBO J. 1984 Oct;3(10):2393–2397. doi: 10.1002/j.1460-2075.1984.tb02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Schwartz M. Expression of a gene in a 400-base-pair fragment of colicin plasmid ColE2-P9 is sufficient to cause host cell lysis. J Bacteriol. 1983 Oct;156(1):109–114. doi: 10.1128/jb.156.1.109-114.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P. The immunity and lysis genes of ColN plasmid pCHAP4. Mol Gen Genet. 1988 Feb;211(2):335–341. doi: 10.1007/BF00330613. [DOI] [PubMed] [Google Scholar]
- Scandella C. J., Kornberg A. A membrane-bound phospholipase A1 purified from Escherichia coli. Biochemistry. 1971 Nov 23;10(24):4447–4456. doi: 10.1021/bi00800a015. [DOI] [PubMed] [Google Scholar]
- Strauch K. L., Beckwith J. An Escherichia coli mutation preventing degradation of abnormal periplasmic proteins. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1576–1580. doi: 10.1073/pnas.85.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Hayashi S., Wu H. C. Synthesis and export of the outer membrane lipoprotein in Escherichia coli mutants defective in generalized protein export. J Bacteriol. 1988 Sep;170(9):4001–4007. doi: 10.1128/jb.170.9.4001-4007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C., Tokunaga M. Biogenesis of lipoproteins in bacteria. Curr Top Microbiol Immunol. 1986;125:127–157. doi: 10.1007/978-3-642-71251-7_9. [DOI] [PubMed] [Google Scholar]