Abstract
Members of the tumor necrosis factor receptor (TNFR) superfamily are important for cell growth and survival. In addition to providing costimulatory signals for cell proliferation, ligation of both TNFR1 and Fas can result in programmed cell death or apoptosis. The underlying mechanism requires an intact 80-aa stretch present in the cytoplasmic tails of both TNFR1 and Fas, termed the death domain (DD). Here we show that CD27, a member of the TNFR family, expressed on discrete subpopulations of T and B cells and known to provide costimulatory signals for T and B cell proliferation and B cell Ig production, can also induce apoptosis. Co-crosslinking of surface Ig receptors along with ligation of CD27 augments CD27-mediated apoptosis. Unlike TNFR1 and Fas, the cytoplasmic tail of CD27 is relatively short and lacks the DD. Using the yeast two-hybrid system, we have cloned a novel protein (Siva) that binds to the CD27 cytoplasmic tail. It has a DD homology region, a box-B-like ring finger, and a zinc finger-like domain. Overexpression of Siva in various cell lines induces apoptosis, suggesting an important role for Siva in the CD27-transduced apoptotic pathway.
CD27 is a member of the tumor necrosis factor receptor (TNFR) superfamily, which includes TNFR types I and II (CD120a and -b), nerve growth factor receptor (NGFR), CD30 (associated with Hodgkin lymphoma), Fas/Apo-1 (CD95), CD40, 4–1BB, and OX40. These receptors are known to play a very important role in cell growth and differentiation, as well as apoptosis or programmed cell death (1). The homology is restricted to the extracellular region of the family members and is characterized by the presence of a Cys knot motif (2), which occurs three times in CD27. CD27 is a glycosylated, type I transmembrane protein of about 55 kDa and exists as homodimers with a disulfide bridge linking the two monomers. The disulfide bridge is in the extracellular domain close to the membrane (3, 4). The ligand for CD27 is CD70, which belongs to the TNF family of ligands. Unlike CD27, CD70 is a type II transmembrane protein with an apparent molecular mass of 50 kDa (5, 6). Because of CD70’s homology to TNFα and -β, especially in β strands C, D, H, and I, CD70 is predicted to have a trimeric structure, made up of three identical subunits, possibly interacting with three CD27 homodimers (7). TNFα is also a type II transmembrane protein and is released to the outside by proteolytic cleavage, whereas TNFβ and NGF are secreted. So far, there are no reports as to the existence of a naturally occurring, soluble form of CD70.
Expression of both CD27 and its ligand, CD70, is restricted to discrete populations of both T and B cells. Although CD27 is expressed on the surface of resting T cells, CD70 appears only on activated T and B cells (8–11). Within the T cell subsets, CD27 is stably expressed on the CD45RA+ population of T cells even after activation, whereas on CD45RO+ cells, it is weakly expressed and lost after activation (8, 9). On CD45RA+ cells, activation by various means results in the up-regulation of CD27 expression (9, 12). Although CD70 is not detectable on either CD45RA+ or CD45RO+ resting T cells, activation through the TcR–CD3 complex results in the expression of CD70 predominantly on CD45RO+ CD4+ T cells. The reciprocal expression of CD27 and CD70 on subsets of helper cells suggested an important role for the molecules in T cell–T cell interactions, T cell activation, and regulation of Ig synthesis. Significant amounts of CD27 can also be detected on a subpopulation of B cells present in peripheral blood and tonsils (12), and the expression can be enhanced after activation with phorbol 12-myristate 13-acetate/ionomycin. CD27 is also expressed on the CD3-bright thymocytes and can be induced in low CD3, CD4+, and CD8+ (double-positive) cells after activation with ConA and phorbol 12-mysristate 13-acetate/ionomycin (13). In contrast, in murine systems, CD27 is constitutively expressed on all thymocytes (14). A soluble form of CD27 (with the extracellular region clipped by a protease) appears in the culture supernatant and can also be detected in the serum of normal individuals (15). CD27 is also highly expressed in most of the B cell non-Hodgkin lymphomas and B cell chronic lymphocytic leukemias (16, 17). The B cell lines Ramos and Raji express significant levels of both CD27 and its ligand, CD70.
Ligation of CD27 along with treatment of T cells with a suboptimal dose of phorbol 12-mysristate 13-acetate, phytohemagglutinin, or anti-CD2 or anti-CD3 antibodies results in the proliferation of T cells, thus defining a costimulatory role for CD27. The CD27-mediated costimulatory effect can be specifically inhibited by the addition of anti-CD27 antibody, or recombinant sCD27 (soluble) or anti-CD70 antibody (8, 9, 11, 18). CD27/CD70 interaction can also result in the generation of cytolytic T cells (5). Ligation of CD27 with CD70 on B cells significantly enhances IgG production, with a less pronounced effect on cell proliferation (19). These studies clearly emphasize the importance of CD27/CD70 binding in T cell–T cell and T cell–B cell interactions. Unlike CD28-mediated T cell proliferation, CD27-mediated T cell proliferation does not support secretion of large amounts of interleukin (IL)-2, clearly defining a different role for CD27/CD70 coupled costimulatory pathways. The CD45RA+ T cells that express CD27 are poor producers of IL-2 and -4 (8), as opposed to CD28, for which coligation with the TcR–CD3 complex results in elevated levels of IL-2.
Here we demonstrate that CD27 may play a role in apoptosis through its association with Siva, a novel CD27 cytoplasmic tail (CT)-binding protein with proapoptotic properties.
MATERIALS AND METHODS
Plasmids and Transfectants.
The generation of mock human CD27 and human CD70 transfectants in the murine pre-B 300–19 cell line has been described (18, 19); these transfectants were kindly provided by C. Morimoto (Dana–Farber Cancer Institute). CD27CT (residues 192–240) was subcloned in frame at the 3′ end of the Gal4 DNA binding domain of the yeast shuttle vector pAS2 at the NdeI and BamHI sites. CD27WT (wild type, full length) was subcloned into the RcCMV vector at the HindII and XbaI sites. A Siva sequence (residues 1–567) was fused in frame to the C terminus of green fluorescent protein (GFP) using the EcoRI site in the pEGFPC1 plasmid (CLONTECH). Because PCR was used to generate specific restriction sites, all the fusions constructed were confirmed by sequencing the coding frame. pAS2LMP1 and pGADLap1 were the kind gift of E. Kieff (Brigham and Women’s Hospital, Boston).
Yeast Two-Hybrid Screening.
The procedures followed were essentially according to the manufacture’s instructions (CLONTECH manual). The yeast strain HF7C was transfected with pAS2CD27CT and pGADGH containing the HeLa cDNA library. Potential CD27CT-interacting clones were selected based on their ability to grow in the absence of Leu, Trp, and His and for their capacity to turn blue in the presence of 5-bromo-4-chloro-3-indolyl β-d-galactosidase (X-Gal). Positive colonies were picked, and the library-derived plasmids were recovered by transforming HB101 bacteria grown on minimal plates in the presence of ampicillin and absence of Leu. The interacting clones were confirmed by retransfecting HF7C along with the pAS2CD27CT. A GenBank search with the DNA and protein sequence of H2 confirmed that it was novel. The H2 insert was then used to screen a HeLa cDNA (λzap1) and a 5′ stretch human thymus cDNA (λgt11) libraries to obtain the full-length Siva cDNA. All the vectors and libraries were purchased from CLONTECH.
Northern Blot Analysis and Transient Transfections.
Premade Northern blots [2 μg of poly(A)+ RNA per lane] representing various human tissues and cell lines (CLONTECH) were probed with the H2 insert labeled with [32P]ATP using the Quick Prime kit (Pharmacia). Both prehybridization and hybridization were carried out at 42°C in the presence of formamide. Suspension cells were transfected using the Lipofectamine reagent (Life Technologies, Gaithersburg, MD). Adherent cells were transfected using the calcium phosphate procedure. Transfection efficiencies with both procedures ranged from 15% to 20%.
DNA Fragmentation Assay.
Cells were lysed (0.4% Triton X-100/4 mM EDTA in 25 mM Tris, pH 7.5). The supernatant was treated with phenol:chloroform and the DNA was precipitated. Before it was separated on agarose, DNA was treated with RNase.
Immunoprecipitations and Western Blotting.
293 cells (transformed, human embryonal kidney cells) were detached with use of PBS containing 2 mM EDTA, collected, and lysed (1% Nonidet P-40 and 150 mM NaCl in 20 mM Tris, pH 7.5) in the presence of protease inhibitors. The supernatant was precleared with anti-mouse Ig coupled to Sepharose beads, and immunoprecipitation was carried out with use of anti-CD27 monoclonal antibody (1A4, 3 μg/ml; a kind gift of C. Morimoto, Dana–Farber Cancer Institute) and anti-mouse IgG beads. Whole-cell lysates were prepared using radioimmunoprecipitation assay buffer (1% Nonidet P-40/1% sodium deoxycholate/0.1% SDS). Proteins were separated on an SDS/12% polyacrylamide gel, transferred to a nitrocellulose membrane, blocked with 3% BSA, and immunoblotted with anti-GFP rabbit antiserum (1:1000), and developed with use of the enhanced chemiluminescence reagent.
RESULTS
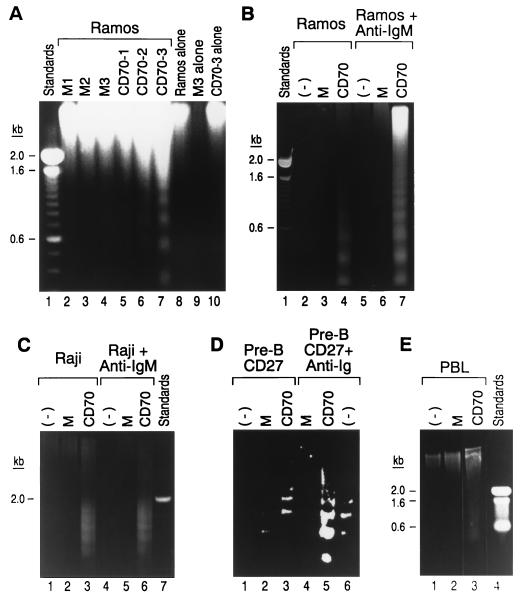
Although several of the TNFR family members are known to induce apoptosis (1), there has been no report to date as to whether CD27 is capable of effecting this cell function. To test this, Ramos, a B cell line that expresses high levels of surface CD27, was cultured at relatively low cell density for 2 days with varying concentrations of live CD70 or mock transfectants. Apoptosis was only observed after culture in the presence of CD70 transfectants, as assessed by DNA fragmentation (Fig. 1A, lanes 6 and 7 vs. lanes 3 and 4). Under similar conditions, no fragmented DNA was observed when Ramos, mock, and CD70 transfectant cells were cultured alone (Fig. 1A, lanes 8–10). To be certain that CD27-bearing cells and not the transfectant cells were undergoing apoptosis, we repeated the experiment using the same transfectants fixed with formaldehyde. Once again, prefixed CD70 transfectants, but not the mock transfectants, were capable of inducing apoptosis in Ramos (Fig. 1B, lane 4 vs. lane 3), Raji, and another B cell line (Fig. 1C, lane 3 vs. lane 2), although this effect was less pronounced using the CD27 transfectant (Fig. 1D, lane 3 vs. lane 2). Prefixing the transfectants and/or culturing the cells alone did not result in DNA fragmentation (data not shown). Because ligation of surface IgM in activated B cells is also known to cause cell death (20, 21), we investigated whether coligation of CD27 would enhance this effect and found that coculture with CD70 transfectants and ligation of surface Ig receptors resulted in marked enhancement in cell death in Ramos (Fig. 1B lane 7 vs. lane 4) and CD27 transfectant (Fig. 1D, lane 5 vs. lane 3) but was not apparent in Raji cells (Fig. 1C, lane 6 vs. lane 3). Ligation of CD27 induces apoptosis in normal human peripheral blood lymphocytes preactivated with phytohemagglutinin and IL-2 (Fig. 1E, lane 3 vs. lane 2).
Figure 1.
Ligation of CD27 induces apoptosis. (A) Ramos cells (0.5 × 106/ml) were cultured with increasing concentrations (0.1, 0.2, and 0.5 × 106/ml) of mock (lanes 2–4) and CD70 transfectants (lanes 5–7) for 2 days. Ramos, mock, and CD70 transfectants when cultured alone are shown (lanes 8–10). (B–E) Experiments with formaldehyde-fixed CD70 and mock transfectants, using Ramos, Raji, human CD27 transfected mouse pre-B 300 cell lines and human peripheral blood lymphocytes preactivated with phytohemagglutinin/IL-2. The effect of coligation of surface IgM (Ramos and Raji) and Ig (murine pre-B 300) using anti-human IgM and anti-mouse IgG antibodies (10 μg/ml), respectively, on CD27-induced apoptosis is also shown (B, lanes 7 and 4; C, lane 6 vs. lane 3; and D, lane 5 vs. lane 3).
The CD27CT is highly conserved between man and mouse (3, 4), suggesting its importance for the receptor function. In this study, we used the CD27CT as the bait and screened a HeLa cell cDNA library using the yeast two-hybrid system. Of the several positive clones, a GenBank search identified the clone H2 as novel. The Epstein–Barr virus transforming protein, LMP1, and its binding protein, Lap1 (TRAF3/CRAF) coding plasmids, were used as the positive control in the yeast system (22). Transfection of the yeast strain HF7C with pAS2LMP1 and pGADLap1 plasmids gave several robust colonies when selected on plates lacking Leu, Tyr, and His, and all of them turned blue in the presence of X-Gal. The clone H2, cotransfected with the CD27CT plasmid, gave several slow-growing slightly smaller size colonies that turned blue in the presence of X-Gal but not when transfected together with the LMP1 plasmid, clearly suggesting the preferential interaction between the CD27CT and H2 (Table 1).
Table 1.
Growth and induction of β-galactosidase in the yeast strain HF7C in the absence of Leu, Trp, and His
| Cotransfection plasmid | pAS2LMP1
|
pAS2CD27CT
|
||
|---|---|---|---|---|
| Colonies | Blue color | Colonies | Blue color | |
| pGADLAP1 | ++++ | ++++ | None | — |
| pGADH2* | None | — | ++ | +++ |
H2* related clones H1 and H7 also gave similar results. pAS2CD27CT and pGADH2 transfectants grown in the absence of Trp and Leu, respectively, did not turn blue in the presence of X-Gal.
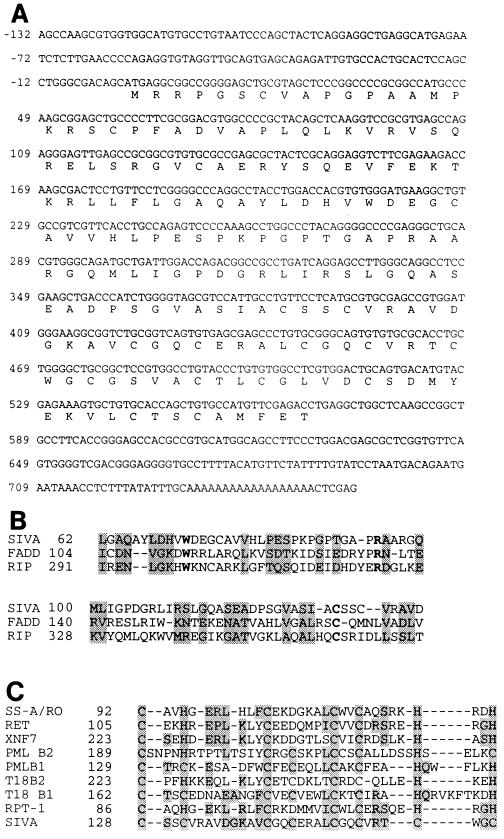
Full-length cDNA sequence was obtained by further screening HeLa cell and human thymocyte cDNA libraries, with use of the H2 insert from the pGADH2 plasmid. Translation of the ORF revealed the primary sequence to be 189 aa long (Fig. 2A); we termed this sequence Siva (after the Hindu god of destruction). Analysis of the primary amino acid sequence revealed an amino-terminal region that has significant homology (40%) to the DDs of FADD and RIP (Fig. 2B). The alignment and homology calculations were determined with use of the program clustal, in which the amino acid size is also taken into consideration. Identical homologies between the Siva and TRADD/Ankyrin DDs was 16% and decreased to 14% when compared with Fas and FADD DDs (data not shown) (23).
Figure 2.
(A) Siva cDNA sequence obtained from HeLa and human thymus cDNA libraries with the translated ORF (189 aa) is shown. The initiation codon is defined by the upstream in-frame stop codon (−42). The coding region is followed by a stop codon (568), a polyadenylylation signal (709–714). The sequence has been deposited in the GenBank under the accession number U2938. (B) A death domain (DD) homology region toward the amino terminus has been identified in Siva, and its homology to FADD and RIP DDs was determined with use of the multiple sequence alignment program clustal. The shaded regions show homology and the residues in boldface type depict identical residues conserved among the three regions. (C) Putative B-box domain of Siva. Homologous residues between Siva and other proteins in the B-box domain are shaded.
The carboxyl-terminal region of Siva is rich in Cys residues and can potentially form a B-box-like ring finger (Fig. 2C, residues 128–159) (24). The B-box region of Siva, however, lacks any His residues. The amino-terminal ring finger and the carboxyl-terminal coiled-coil domain structures, which are characteristic of other B-box-containing proteins (24), are absent in Siva. Instead, Siva is flanked by additional Cys residues in the carboxyl terminus that can potentially form a zinc finger (residues 164–184), which also lacks His. Alternately, the Cys-rich region of Siva could represent a novel metal binding motif involved in either protein–protein or protein–DNA interactions. The architecture of Siva is unlike any of the molecules so far known to bind to the cytoplasmic tails of other TNFR family members.
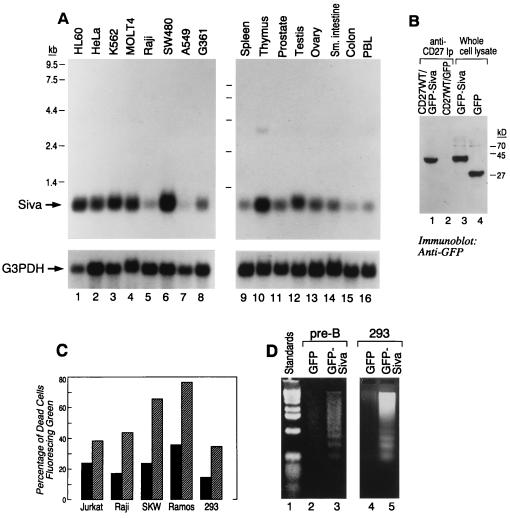
Multiple-human-tissue Northern blot analysis revealed the presence of varying amounts of 0.8 kb Siva mRNA in all the represented tissues. Maximum mRNA expression was seen in thymus, and the least was seen in colon (Fig. 3A Right, upper bands). Several cell lines, including HeLa and Raji, also expressed the mRNA (Fig. 3A Left, upper bands). Relative levels of Siva mRNA expression can be judged from the levels of G3PHD RNA (Fig. 3A, lower bands).
Figure 3.
(A) mRNA expression of Siva by Northern blot analysis is shown (upper bands) and is compared with the expression of G3PDH (lower bands). (B) CD27 binds to Siva in 293 cells. CD27WT DNA was transiently coexpressed with either GFP or GFP–Siva for 2 days. The CD27 receptor expression appeared to be similar as seen by flow cytometric analysis (data not shown). CD27 immunoprecipitates using anti-CD27 antibody (IA4) were separated by SDS/PAGE, transferred, and blotted with anti-GFP antiserum. Similar amounts of GFP and GFP–Siva were expressed as seen in the lanes corresponding to whole-cell lysates (lanes 4 and 3). CD27 coprecipitaed GFP–Siva (lane 1) but not GFP (lane 2). (C) Siva-induced apoptosis. Jurkat, Raji, SKW, Ramos, and 293 cells were transiently transfected (2 days) with GFP (hatched bar) and GFP–Siva (solid bar). The histograms represent the percentage of dead cells (based on cell morphology) in the cells expressing green fluorescence. In the case of 293 cells, along with GFP and GFP–Siva, pSVb containing the β-galactosidase gene was cotransfected. β-Galactosidase expression was visualized by X-Gal. The percentage of rounded shrunken cells in the fluorescent population of cells is presented. (D) Transient expression of GFP–Siva but not GFP can induce apoptosis in 293 cells (lane 3 vs. lane 2), the murine pre-B cell line (lane 6 vs. lane 5), and Raji cells (lane 8 vs. lane 7), as judged by the generation of DNA ladder.
Although the yeast two-hybrid system suggests a direct interaction between the CD27CT and Siva, we confirmed the association between the two by transiently expressing (2 days) CD27WT (full length) and Siva fused to the bacterial GFP (GFP–Siva) in 293 embryonal renal cells. Cell lysates were prepared with use of mild non-ionic detergent such as Nonidet P-40, and the CD27 receptor complexes were immunoprecipitated with use of anti-CD27 monoclonal antibody (1A4). The expression of GFP–Siva and GFP were similar, as seen from the anti-GFP immunoblot (Fig. 3B, lanes 3 and 4). CD27 coprecipitates GFP–Siva but not GFP (Fig. 3B, lanes 1 and 2).
Because CD27 induces cell death and because its binding protein, Siva, has a region with significant homology to the DDs, we wanted to see whether overexpression of Siva can cause significant apoptosis. We transiently expressed GFP and GFP–Siva in various cell lines for 2 days and measured the percentage of dead cells that were fluorescing green. In general, most of the cells expressing GFP–Siva looked unhealthy, were very small, and had an irregular cell border when compared with cells expressing GFP (data not shown). In every cell line tested, the percentage of GFP–Siva-transfected dead cells was 2–3 times higher when compared with those transfected with GFP alone (Fig. 3C). In the case of the adherent cell line 293, we also expressed the β-galactosidase gene along with GFP and GFP–Siva and examined the cell morphology using the light microscope. Most of the cells expressing GFP–Siva were much smaller and rounded (devoid of the cellular processes), clearly suggesting apoptosis. That this indeed is the case was confirmed by a DNA fragmentation assay in 293 cells (adherent) and the murine pre-B cell line (suspension) (Fig. 3D, lane 3 vs. lane 2 and lane 5 vs. lane 4). Electron microscopy performed on GFP- and GFP–Siva-transfected 293 cells revealed the presence of cells with apoptotic bodies only in GFP–Siva transfectants (data not shown).
DISCUSSION
The fact that CD27, a member of the TNFR superfamily, can induce programmed cell death in certain types of cells is not surprising because TNFR family members, although multifarious in terms of the repertoire of functions they exhibit, are also viewed as principal regulators of cell death (1). Both Fas and TNFR1, whose signaling pathways related to apoptosis have been extensively studied, also provide costimulatory signals for growth (25–27). Similarly, the ligation of CD27 in resting T cells results in important costimulatory signals, cell growth, and proliferation. (8, 9, 11, 18). In the case of B cells, CD27 clearly plays a role in B cell differentiation. Engagement of CD27 in the presence of Staphylococcus aureus Cowan I and IL-2 results in slightly increased B cell proliferation, enhanced Ig synthesis (19), and increases the lineage of cells showing a plasma cell phenotype (28). We show above that, in addition to these growth-promoting functions, engagement of CD27 can also result in apoptosis. Interaction between CD27 and CD70 is sufficient to elicit the apoptotic function of CD27. In the absence of crucial complementary signals, it is quite likely that engagement of CD27, especially in activated T and B cells that express CD27 and CD70, may result in apoptosis. Perhaps, in situations of chronic activation, CD27-induced apoptosis may play an important role in maintaining self-tolerance and keeping in check activated T and B cells, which may contribute to some autoimmune diseases. In the case of multiple sclerosis, where elevated levels of sCD27 have been reported, it is possible that these elevations may inhibit the regulatory or other effects of CD70-expressing T and B cells (15).
In some cell lines, we find that co-crosslinking of surface Ig receptors augments CD27-mediated apoptosis. However, Lens et al. (29) recently reported that anti-CD27 antibody had no effect on anti-IgM antibody-induced apoptosis in Ramos cells. In our hands, the apoptotic function of CD27 could only be demonstrated using natural ligand-transfected cells and not by receptor crosslinking with anti-CD27 antibodies (1A4 and 8H5) (unpublished results). Compared with Fas-induced (2–4 h) and TNFR1-induced (8–12 h) apoptosis, the kinetics of CD27-induced cell death appears to be much slower (2 days) (25–27). The B cell lines Ramos and Raji express on their surface relatively high levels of both CD27 and CD70 but do not undergo apoptosis under normal cell culture conditions. This could be due to the relatively low cell density used for routine cell culture, which may not permit the interaction between CD27 and CD70 to reach the requisite threshold. In the experimental conditions reported here, we increased the amount of available CD70 to Ramos cell cultures by several orders of magnitude, thus overcoming the threshold barrier and resulting in apoptosis of CD27-bearing cells. This is supported by the observation that Ramos or Raji cells grown to relatively high cell density (>1.0 × 106 cells per ml) result in increased background apoptosis even in the presence of optimum amounts of nutrients. B cell cancers such as non-Hodgkin lymphoma and B-CLL do not undergo apoptosis, despite the high expression of both CD27 and CD70. This could be due to the fact that these cells release sCD27 (16, 17), which builds up in the body fluids and possibly disrupts CD27-mediated apoptosis. A similar finding has been reported for the poxvirus gene products T2 and A536, which are soluble secreted forms of TNFR; they can bind to TNF produced by host cells and may interfere in the normal functioning of TNF (30). Disruption of the binding between CD27 and CD70 by sCD27 is also likely to aid in metastasis, thus playing an accelerating role in the progress of these cancers.
Although significant homology is observed in the extracellular regions of the various TNFR family members, the cytoplasmic tails of the various members appear to be distinct. However, TNFRI and Fas have the DD in their cytoplasmic tails, a location that is essential for induction of apoptosis through these receptors (31, 32). The DD spans a stretch of about 80 aa. CD27 has relatively a short cytoplasmic tail (49 aa) and therefore lacks a DD. Because CD27CT is highly conserved between man and mouse, it is likely to be required for CD27-mediated cellular functions. From these observations, we surmised that there may be a cytoplasmic protein which mediates the apoptotic signal initially transduced by CD27. Accordingly, we used the human CD27CT as the bait in the yeast two-hybrid system and were able to identify a novel CD27-interacting protein, which we termed Siva.
Thus far, two types of molecules that interact with various TNFR family members have surfaced. One group comprises the DD-containing proteins TRADD, FADD, and RIP, which interact with Fas and TNFRI (33–36). TRAFs form the second group, characterized by the zinc finger domains, and interact with TNFRII, CD40, and LMP1 (22, 37–39). In comparison, Siva does not appear to fall into either of these categories. Using the clustal program, which takes into consideration both the size and hydrophobicity of amino acids, a region in Siva homologous to the known DDs of FADD and RIP was identified. Although, overall homology between the three is high, identical homology is low. The dendrogram analysis clearly places Siva outside all of the known DD-containing proteins (data not shown). The homologies reported here are comparable to those calculated for TRADD/FADD and TRADD/RIP and are higher than those for the DD of Reaper and other DD-containing proteins (23). NMR structure of Fas DD revealed the presence of six antiparallel, amphipathic α-helices, and a similar structure has been proposed for other DDs (40). However, based on secondary structure predictions, the DD homology region of Siva appears to lack at least four of these helices and thus could possibility be structurally different from that of the DD of Fas and its distant relative Reaper. An important consideration is that all DDs do not appear to be similar in terms of cellular function. For example, the DD of FADD is required for binding of FADD to the FAS DD but not for induction of apoptosis (35, 41). The TRADD DD, however, is required for eliciting both apoptosis and activation of the transcription factor NFkB (34, 42). In case of the Drosophila protein Reaper, mutations carried out in the DD region similar to that of Fas DD do not abrogate the potent apoptotic activity of the protein (43). It therefore appears that DDs are multipurpose protein-interacting domains. The DD homology region of Siva appears to be distantly related to other DDs, and its function is currently under investigation.
The Cys-rich carboxyl terminus of Siva can be viewed as a combination of both B-box- and zinc finger-like domains (24), but the conserved His is missing in Siva. Because all of this region is present in the original yeast insert H2, this region probably mediates the binding to CD27CT. Carboxyl-terminal zinc fingers are known to be involved in mediating protein–protein interactions (24). Support for assigning the binding domain to the zinc finger domain is provided by the report that the EGFR constitutively binds through its cytoplasmic tail to the zinc finger protein ZPR1, where association is mediated through the zinc finger domain (44).
Using an approach similar to that reported for FADD, TRADD, and RIP (33–35), by transiently expressing Siva as a GFP fusion protein, we could demonstrate its proapoptotic properties in various cell lines, both by morphology and DNA fragmentation assays. Further substantiation came from propidium iodide staining of 293 cells and analyzing the percentage of sub-G1 cells fluorescing green, corresponding to the apoptotic population in GFP transfectants. For 293 cells transfected with GFP–Siva, the percentage of apoptotic cells was 2–3 times higher than those transfected with GFP alone (data not shown). This clearly suggests an important role for Siva in CD27-mediated signaling pathway. The existence of CD27–Siva protein complexes in vivo and the role of Siva in CD27-mediated apoptosis under physiological conditions remains to be established.
Acknowledgments
We thank Drs. E. Kieff, A. Bharti, M. R. Rao, G. Mosialos, K. Sastry, M. Sastry, R. Vadlamudi, J. Shin, D. Weaver, and H. Saito for their helpful advice. K.V.S.P. is the recipient of Claudia Adams Barr Award.
ABBREVIATIONS
- TNFR
tumor necrosis factor receptor
- NGFR
nerve growth factor receptor
- CD27CT
cytoplasmic tail of CD27
- DD
death domain
- X-Gal
5-bromo-4-chloro-3-indolyl β-d-galactosidase
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. U2938).
References
- 1.Smith C A, Farrah T, Goodwin R G. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 2.McDonald N Q, Hendrickson W A. Cell. 1993;73:421–424. doi: 10.1016/0092-8674(93)90127-c. [DOI] [PubMed] [Google Scholar]
- 3.Camerini D, Walz G, Loenen W A M, Borst J, Seed B. J Immunol. 1991;147:3165–3169. [PubMed] [Google Scholar]
- 4.Gravestein L A, Bloom B, Nolten L A, Van Der Vries E. Eur J Immunol. 1993;23:943–950. doi: 10.1002/eji.1830230427. [DOI] [PubMed] [Google Scholar]
- 5.Goodwin R G, Alderson M R, Smith C A, Armitage R J, Vanden Bos T, Jerzy R, Tough T W, Schoenborn M J, Davis-Smith T, Hennen K, Falk B, Cosman D, Baker E, Sutherland G R, Grabstein K H, Farrah T, Giri J G, Beckmann P M. Cell. 1993;73:447–456. doi: 10.1016/0092-8674(93)90133-b. [DOI] [PubMed] [Google Scholar]
- 6.Bowman M R, Crimmins M A V, Yetz-Aldape J, Kriz R, Kelleher K, Herrman S. J Immunol. 1994;1521:1756–1761. [PubMed] [Google Scholar]
- 7.Peitsch M C, Tschopp J. Mol Immunol. 1995;32:761–772. doi: 10.1016/0161-5890(95)00016-8. [DOI] [PubMed] [Google Scholar]
- 8.Sugita K, Hirose T, Rothstein D M, Donahue C, Schlossman S F, Morimoto C. J Immunol. 1992;149:3208–3216. [PubMed] [Google Scholar]
- 9.Hintzen R Q, De Jong R, Lens S M A, Brouwer M, Baars P, Van Lier R A W. J Immunol. 1993;151:2426–2435. [PubMed] [Google Scholar]
- 10.Agematsu K, Kobata T, Sugita K, Freeman G J, Beckmann M P, Schlossman S F, Morimoto C. J Immunol. 1994;153:1421–1429. [PubMed] [Google Scholar]
- 11.Hintzen R Q, Lens S M A, Lammers K, Kuper H, Beckmann P, Van Lier R A W. J Immunol. 1995;154:2612–2623. [PubMed] [Google Scholar]
- 12.Maurer D, Holter W, Majdic O, Fischer G F, Knapp W. Eur J Immunol. 1990;20:2679–2684. doi: 10.1002/eji.1830201223. [DOI] [PubMed] [Google Scholar]
- 13.Martorell J, Rojo I, Viella R, Martinez-Caceres E, Vives J. J Immunol. 1990;145:1356–1363. [PubMed] [Google Scholar]
- 14.Gravestein L A, Nieland J D, Kruisbeek A M, Borst J. Int J Immunol. 1994;7:551–557. doi: 10.1093/intimm/7.4.551. [DOI] [PubMed] [Google Scholar]
- 15.Hintzen R Q, Van Lier R A W, Kuijpers K C, Baars P A, Schaasberg W, Lukas C J, Polman C H. J Neuroimmunol. 1991;35:211–218. doi: 10.1016/0165-5728(91)90175-7. [DOI] [PubMed] [Google Scholar]
- 16.Ranheim E A, Cantwell M J, Kipps T J. Blood. 1995;85:3556–3565. [PubMed] [Google Scholar]
- 17.Van Oers M H, Pals S T, Evers L M, Van der Schoot C E. Blood. 1993;82:3430–3436. [PubMed] [Google Scholar]
- 18.Kobata T, Agematsu K, Kameoka J, Schlossman S F, Morimoto C. J Immunol. 1994;153:5422–5432. [PubMed] [Google Scholar]
- 19.Kobata T, Jacquot S, Kozlowski S, Agematsu K, Schlossman S F, Morimoto C. Proc Natl Acad Sci USA. 1995;92:11249–11253. doi: 10.1073/pnas.92.24.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsubata T, Wu J, Honjo T. Nature (London) 1993;364:645–648. doi: 10.1038/364645a0. [DOI] [PubMed] [Google Scholar]
- 21.Valentine M A, Licciardi K A. Eur J Immunol. 1992;22:3141–3148. doi: 10.1002/eji.1830221217. [DOI] [PubMed] [Google Scholar]
- 22.Mosialos G, Birkenbach M, Yalamanchili R, Van Arsdale T, Ware C, Kieff E. Cell. 1995;80:389–399. doi: 10.1016/0092-8674(95)90489-1. [DOI] [PubMed] [Google Scholar]
- 23.Cleveland J L, Ihle J N. Cell. 1995;81:479–482. doi: 10.1016/0092-8674(95)90068-3. [DOI] [PubMed] [Google Scholar]
- 24.Freemont P S. Ann N Y Acad Sci. 1993;684:174–192. doi: 10.1111/j.1749-6632.1993.tb32280.x. [DOI] [PubMed] [Google Scholar]
- 25.Alderson M R, Armitage R J, Maraskovsky E, Tough T W, Roux E, Schooley K, Ramsdell F, Lynch D H. J Exp Med. 1993;178:2231–2235. doi: 10.1084/jem.178.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tartaglia L A, Rothe M, Hu Y F, Goeddel D V. Cell. 1993;73:213–216. doi: 10.1016/0092-8674(93)90222-c. [DOI] [PubMed] [Google Scholar]
- 27.Suda T, Takahashi T, Nagata S. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 28.Jacquot S, Kobata T, Iwata S, Morimoto C, Schlossman S F. In: In Leucocyte Typing VI. Kishimoto T, Gioyert S, Kikutani H, Mason D, Miyasaka M, Moretta L, Ohno T, Okumura K, Shaw S, Springer T A, Sugamura K, Sugawara H, Von Dem Borne A E G K, Zola H, editors. New York: Garland; 1997. , in press. [Google Scholar]
- 29.Lens S A, Tesselaar K, Drijver B F A, Van Oers M H J, Van Lier A W. J Immunol. 1996;156:507–514. [PubMed] [Google Scholar]
- 30.Smith C, Davis T, Wignall J, Din W, Farrah T, Upton C, McFadden G, Goodwin R. Biochem Biophys Res Commun. 1991;176:335–342. doi: 10.1016/0006-291x(91)90929-2. [DOI] [PubMed] [Google Scholar]
- 31.Tartaglia L A, Ayres T, M, Wong G H, Goeddel D V. Cell. 1993;74:845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- 32.Itoh N, Nagata S. J Biol Chem. 1993;268:10932–10937. [PubMed] [Google Scholar]
- 33.Hsu H, Xiong J, Goeddel D V. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 34.Chinnaiyan A M, O’Rourke K, Tewari M, Dixit V M. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 35.Stanger Z B, Leder P, Lee T, Kim E, Seed B. Cell. 1995;81:513–523. doi: 10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 36.Hsu H, Huang J, Shu H, Baichwal V, Goeddel D. Immunity. 1996;4:387–396. doi: 10.1016/s1074-7613(00)80252-6. [DOI] [PubMed] [Google Scholar]
- 37.Rothe M, Sarma V, Dixit M, Goeddel D V. Science. 1995;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- 38.Hu H M, O’Rourke K, Tewari M, Dixit V M. J Biol Chem. 1994;269:30069–30072. [PubMed] [Google Scholar]
- 39.Cheng G, Cleary A M, Ye Z S, Hong D I, Lederman S, Baltimore D. Science. 1995;267:1494–1498. doi: 10.1126/science.7533327. [DOI] [PubMed] [Google Scholar]
- 40.Huang B, Eberstadt M, Olejniczak E T, Meadows R P, Fesik S. Nature (London) 1996;384:638–641. doi: 10.1038/384638a0. [DOI] [PubMed] [Google Scholar]
- 41.Grimm S, Stanger B Z, Leder P. Proc Natl Acad Sci USA. 1996;93:10923–10927. doi: 10.1073/pnas.93.20.10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park A, Baichwal R. J Biol Chem. 1996;271:9858–9862. doi: 10.1074/jbc.271.16.9858. [DOI] [PubMed] [Google Scholar]
- 43.Chen P, Lee P, Otto L, Abrams J. J Biol Chem. 1996;271:25735–25737. doi: 10.1074/jbc.271.42.25735. [DOI] [PubMed] [Google Scholar]
- 44.Galcheva-Gargova Z, Konstantinov K, Wu I, Klier G, Barrett T, Davis R J. Science. 1995;272:1797–1802. doi: 10.1126/science.272.5269.1797. [DOI] [PubMed] [Google Scholar]