Abstract
The antigenically variant M protein of Streptococcus pyogenes enhances virulence by promoting resistance to phagocytosis. The serum opacity factor (OF), produced by a subset of M serotypes, is also antigenically variant, and its antigenic variability exactly parallels that of M protein. OF-positive and OF-negative streptococci are also phenotypically distinguishable by a number of other criteria. In order to study the differences between OF-positive and OF-negative streptococci, we cloned and sequenced the type 49 M protein gene (emm49), the first to be cloned from an OF-positive strain. This gene showed evolutionary divergence from the OF-negative M protein genes studied previously. Furthermore, emm49 was part of a gene family, in contrast to the single-copy nature of previously characterized M protein genes.
Full text
PDF

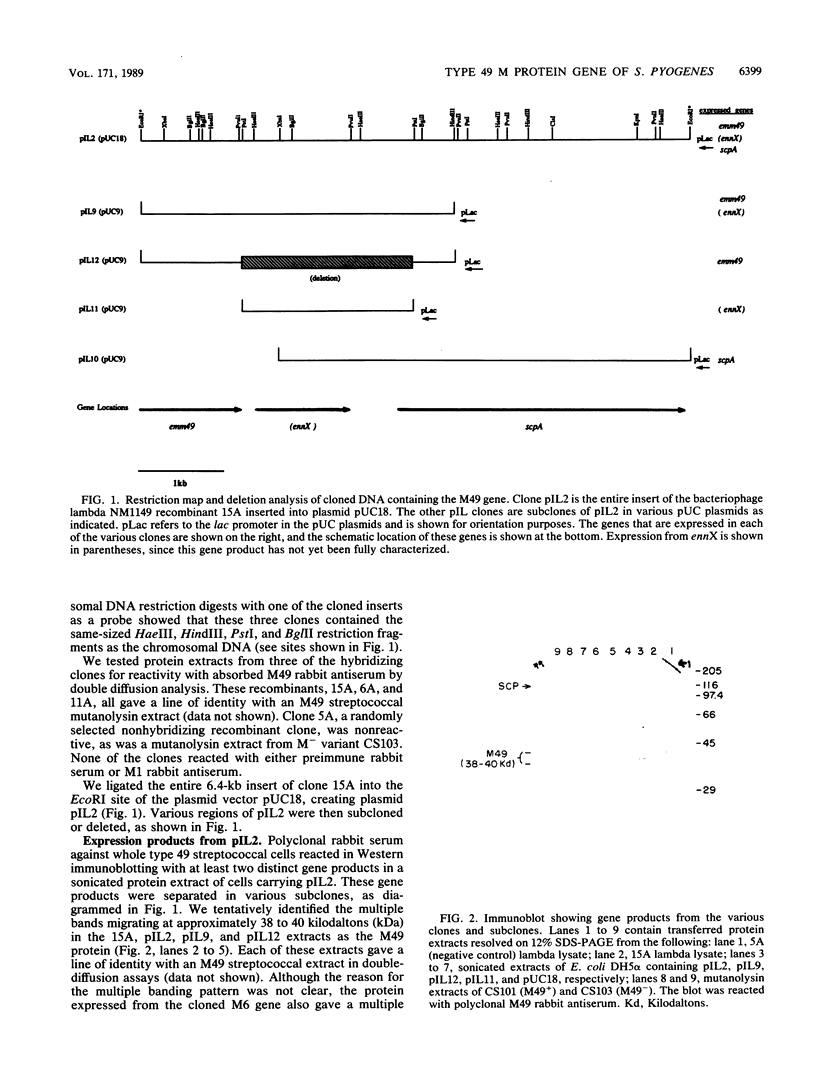




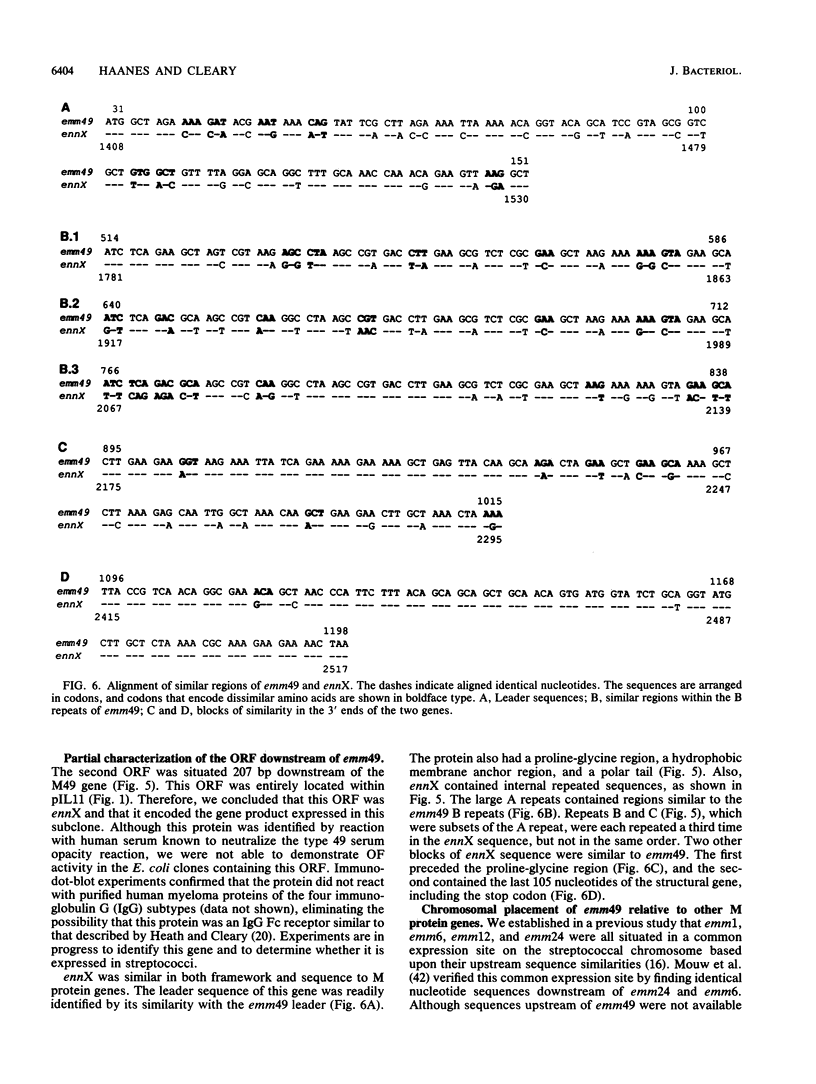




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Erickson B. W. Optimal sequence alignment using affine gap costs. Bull Math Biol. 1986;48(5-6):603–616. doi: 10.1007/BF02462326. [DOI] [PubMed] [Google Scholar]
- Anderson R. P., Roth J. R. Tandem genetic duplications in phage and bacteria. Annu Rev Microbiol. 1977;31:473–505. doi: 10.1146/annurev.mi.31.100177.002353. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bessen D., Jones K. F., Fischetti V. A. Evidence for two distinct classes of streptococcal M protein and their relationship to rheumatic fever. J Exp Med. 1989 Jan 1;169(1):269–283. doi: 10.1084/jem.169.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Cameron J. R., Philippsen P., Davis R. W. Analysis of chromosomal integration and deletions of yeast plasmids. Nucleic Acids Res. 1977;4(5):1429–1448. doi: 10.1093/nar/4.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. C., Cleary P. P. Cloning and expression of the streptococcal C5a peptidase gene in Escherichia coli: linkage to the type 12 M protein gene. Infect Immun. 1989 Jun;57(6):1740–1745. doi: 10.1128/iai.57.6.1740-1745.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary P. P. Genetic separation of serum opacity factor from M protein of group A streptococci. Infect Immun. 1978 Oct;22(1):171–175. doi: 10.1128/iai.22.1.171-175.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Donelson J. E., Rice-Ficht A. C. Molecular biology of trypanosome antigenic variation. Microbiol Rev. 1985 Jun;49(2):107–125. doi: 10.1128/mr.49.2.107-125.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D. F., Doolittle R. F. Progressive sequence alignment as a prerequisite to correct phylogenetic trees. J Mol Evol. 1987;25(4):351–360. doi: 10.1007/BF02603120. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A., Jones K. F., Manjula B. N., Scott J. R. Streptococcal M6 protein expressed in Escherichia coli. Localization, purification, and comparison with streptococcal-derived M protein. J Exp Med. 1984 Apr 1;159(4):1083–1095. doi: 10.1084/jem.159.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Jones K. F., Scott J. R. Size variation of the M protein in group A streptococci. J Exp Med. 1985 Jun 1;161(6):1384–1401. doi: 10.1084/jem.161.6.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner R. C., Howarth A. J., Messing J., Shepherd R. J. Cloning and sequencing of restriction fragments generated by Eco RI*. DNA. 1982;1(2):109–115. doi: 10.1089/dna.1.1982.1.109. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Haanes-Fritz E., Kraus W., Burdett V., Dale J. B., Beachey E. H., Cleary P. Comparison of the leader sequences of four group A streptococcal M protein genes. Nucleic Acids Res. 1988 May 25;16(10):4667–4677. doi: 10.1093/nar/16.10.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagblom P., Segal E., Billyard E., So M. Intragenic recombination leads to pilus antigenic variation in Neisseria gonorrhoeae. Nature. 1985 May 9;315(6015):156–158. doi: 10.1038/315156a0. [DOI] [PubMed] [Google Scholar]
- Hallas G., Widdowson J. P. The relationship between opacity factor and M protein in Streptococcus pyogenes. J Med Microbiol. 1983 Feb;16(1):13–26. doi: 10.1099/00222615-16-1-13. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Heath D. G., Cleary P. P. Cloning and expression of the gene for an immunoglobulin G Fc receptor protein from a group A streptococcus. Infect Immun. 1987 May;55(5):1233–1238. doi: 10.1128/iai.55.5.1233-1238.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath D. G., Cleary P. P. Fc-receptor and M-protein genes of group A streptococci are products of gene duplication. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4741–4745. doi: 10.1073/pnas.86.12.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill M. J., Wannamaker L. W. The serum opacity reaction of Streptococcus pyogenes: general properties of the streptococcal factor and of the reaction in aged serum. J Hyg (Lond) 1968 Mar;66(1):37–47. doi: 10.1017/s0022172400040924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Hollingshead S. K., Fischetti V. A., Scott J. R. Complete nucleotide sequence of type 6 M protein of the group A Streptococcus. Repetitive structure and membrane anchor. J Biol Chem. 1986 Feb 5;261(4):1677–1686. [PubMed] [Google Scholar]
- Hollingshead S. K., Fischetti V. A., Scott J. R. Size variation in group A streptococcal M protein is generated by homologous recombination between intragenic repeats. Mol Gen Genet. 1987 May;207(2-3):196–203. doi: 10.1007/BF00331578. [DOI] [PubMed] [Google Scholar]
- Horstmann R. D., Sievertsen H. J., Knobloch J., Fischetti V. A. Antiphagocytic activity of streptococcal M protein: selective binding of complement control protein factor H. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1657–1661. doi: 10.1073/pnas.85.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks-Weis J., Kim Y., Cleary P. P. Restricted deposition of C3 on M+ group A streptococci: correlation with resistance to phagocytosis. J Immunol. 1982 Apr;128(4):1897–1902. [PubMed] [Google Scholar]
- Jones K. F., Fischetti V. A. Biological and immunochemical identity of M protein on group G streptococci with M protein on group A streptococci. Infect Immun. 1987 Mar;55(3):502–506. doi: 10.1128/iai.55.3.502-506.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. F., Hollingshead S. K., Scott J. R., Fischetti V. A. Spontaneous M6 protein size mutants of group A streptococci display variation in antigenic and opsonogenic epitopes. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8271–8275. doi: 10.1073/pnas.85.21.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe M. A., Poirier T. P., Beachey E. H., Timmis K. N. Cloning and genetic analysis of serotype 5 M protein determinant of group A streptococci: evidence for multiple copies of the M5 determinant in the Streptococcus pyogenes genome. Infect Immun. 1985 Apr;48(1):190–197. doi: 10.1128/iai.48.1.190-197.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus W., Haanes-Fritz E., Cleary P. P., Seyer J. M., Dale J. B., Beachey E. H. Sequence and type-specific immunogenicity of the amino-terminal region of type 1 streptococcal M protein. J Immunol. 1987 Nov 1;139(9):3084–3090. [PubMed] [Google Scholar]
- LANCEFIELD R. C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962 Sep;89:307–313. [PubMed] [Google Scholar]
- LANCEFIELD R. C. Persistence of type-specific antibodies in man following infection with group A streptococci. J Exp Med. 1959 Aug 1;110(2):271–292. doi: 10.1084/jem.110.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MOODY M. D., PADULA J., LIZANA D., HALL C. T. EPIDEMIOLOGIC CHARACTERIZATION OF GROUP A STREPTOCOCCI BY T-AGGLUTINATION AND M-PRECIPITATION TESTS IN THE PUBLIC HEALTH LABORATORY. Health Lab Sci. 1965 Jul;2:149–162. [PubMed] [Google Scholar]
- Maxted W. R. Group A streptococci: pathogenesis and immunity. Soc Appl Bacteriol Symp Ser. 1978;7:107–125. [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Miller L., Gray L., Beachey E., Kehoe M. Antigenic variation among group A streptococcal M proteins. Nucleotide sequence of the serotype 5 M protein gene and its relationship with genes encoding types 6 and 24 M proteins. J Biol Chem. 1988 Apr 25;263(12):5668–5673. [PubMed] [Google Scholar]
- Mouw A. R., Beachey E. H., Burdett V. Molecular evolution of streptococcal M protein: cloning and nucleotide sequence of the type 24 M protein gene and relation to other genes of Streptococcus pyogenes. J Bacteriol. 1988 Feb;170(2):676–684. doi: 10.1128/jb.170.2.676-684.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancholi V., Fischetti V. A. Isolation and characterization of the cell-associated region of group A streptococcal M6 protein. J Bacteriol. 1988 Jun;170(6):2618–2624. doi: 10.1128/jb.170.6.2618-2624.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier T. P., Kehoe M. A., Dale J. B., Timmis K. N., Beachey E. H. Expression of protective and cardiac tissue cross-reactive epitopes of type 5 streptococcal M protein in Escherichia coli. Infect Immun. 1985 Apr;48(1):198–203. doi: 10.1128/iai.48.1.198-203.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. C., Spanier J. G., Jones S. J., Simpson W. J., Cleary P. P. Streptococcus pyogenes type 12 M protein gene regulation by upstream sequences. J Bacteriol. 1987 Dec;169(12):5633–5640. doi: 10.1128/jb.169.12.5633-5640.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. R., Hollingshead S. K., Fischetti V. A. Homologous regions within M protein genes in group A streptococci of different serotypes. Infect Immun. 1986 May;52(2):609–612. doi: 10.1128/iai.52.2.609-612.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh L., Jones K. W. The use of heparin as a simple cost-effective means of controlling background in nucleic acid hybridization procedures. Nucleic Acids Res. 1984 Jul 25;12(14):5627–5638. doi: 10.1093/nar/12.14.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. P. Evolution of repeated DNA sequences by unequal crossover. Science. 1976 Feb 13;191(4227):528–535. doi: 10.1126/science.1251186. [DOI] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Top F. H., Jr, Wannamaker L. W. The serum opacity reaction of Streptococcus pyogenes. The demonstration of multiple, strain-specific lipoproteinase antigens. J Exp Med. 1968 May 1;127(5):1013–1034. doi: 10.1084/jem.127.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Top F. H., Jr, Wannamaker L. W. The serum opacity reaction of Streptococcus pyogenes: frequency of production of streptococcal lipoproteinase by strains of different serological types and the relationship of M protein production. J Hyg (Lond) 1968 Mar;66(1):49–58. doi: 10.1017/s0022172400040936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Widdowson J. P., Maxted W. R., Grant D. L. The production of opacity in serum by group A streptococci and its relationship withthe presence of M antigen. J Gen Microbiol. 1970 Jun;61(3):343–353. doi: 10.1099/00221287-61-3-343. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]



