Abstract
Mast cells synthesize and secrete specific cytokines and chemokines which play an important role in allergic inflammation. Aggregation of the high-affinity Fc receptor (FcɛRI) for immunoglobulin E (IgE) in MC/9 mouse mast cells stimulates the synthesis and secretion of tumor necrosis factor α (TNF-α). FcɛRI aggregation activates several sequential protein kinase pathways, leading to increased activity of extracellular signal-regulated kinases (ERKs), c-Jun amino-terminal kinases (JNKs), and the p38 mitogen-activated protein (MAP) kinase. Inhibition of ERKs with the compound PD 098059 had little effect on FcɛRI-stimulated TNF-α production. Aggregation of FcɛRI stimulated MEK kinase 1 (MEKK1) activity, which activates JNK kinase (JNKK), the kinase that phosphorylates and activates JNKs. Expression of activated MEKK1 (ΔMEKK1) in MC/9 cells strongly stimulated JNK activity but only weakly stimulated p38 activity, and it induced a large activation of TNF-α promoter-regulated luciferase gene expression. Inhibitory mutant JNK2 expressed in MC/9 cells significantly blunted FcɛRI stimulation of TNF-α promoter-driven luciferase expression. Wortmannin, an inhibitor of phosphatidylinositol 3-kinase, diminished FcɛRI-mediated TNF-α synthesis, significantly blunted JNK activation and TNF-α promoter-driven luciferase expression, and only weakly inhibited p38 kinase activation. Inhibition of NFκB activation resulting from ΔMEKK1 expression or FcɛRI stimulation did not affect TNF-α promoter-driven luciferase expression. Our findings define a MEKK-regulated JNK pathway activated by FcɛRI that regulates TNF-α production in mast cells.
Mast cells play a central role in inflammatory and immediate allergic reactions. The multivalent binding of antigen to receptor-bound IgE and the subsequent aggregation of the high-affinity Fc receptors for IgE (FcɛRI) provide the trigger for activation of mast cells. FcɛRI aggregation results in the release of inflammatory mediators, including histamine, leukotriene C4, and prostaglandin D2. In addition to inflammatory mediators being released, several cytokines are produced in mast cells (1–4). The production of cytokines, such as tumor necrosis factor α (TNF-α), is triggered by the aggregation of FcɛRI on the surface of mast cells. TNF-α is critical in the immediate inflammatory response; for example, TNF-α controls the up-regulation of adhesion proteins on endothelial cells for interaction with leukocytes (5, 6). Increased transcription of the TNF-α gene following FcɛRI aggregation is inhibited by the immunosuppressant cyclosporin A, and the tyrosine kinase activity associated with IgE stimulation of the mast cell is also required for cytokine production (7–9). In monocytes, a series of pyridinylimidazole compounds have been shown to block lipopolysaccharide-induced activation of the p38 mitogen-activated protein kinase (MAP kinase), a serine/threonine protein kinase shown to be involved in inflammatory responses. Inhibition of p38 MAP kinase inhibits TNF-α synthesis primarily at the level of translation and expression (10). In addition to the p38 MAP kinase, little is known about the signal transduction pathways, other than the role of calcineurin, that regulate TNF-α gene expression. The regulation of NFATp by calcineurin and transcriptional regulation by κ regulatory elements that bind NFATp in the TNF-α promoter appear pivotal (11–13). Clues are evident from the promoter for the TNF-α gene, which contains NFκB-, AP-1-, AP-2-, NFAT-, Ets-, and AP-1/ATF-related elements for additional signal transduction pathways that regulate TNF-α gene expression (14–20). The regulatory elements in the TNF-α promoter suggest that MAP kinase members including the extracellular signal-regulated kinases (ERKs), c-Jun amino-terminal kinases (JNKs), and p38 MAP kinase, which are known to regulate c-Jun and ATF-2 transcription factors, may be involved in regulating TNF-α gene expression. The MEK kinases are upstream components of the sequential protein kinase pathways regulating the JNKs (21, 22). MEK kinases have also been shown to regulate the activation of NFκB, which has been proposed to be important in controlling TNF-α gene transcription (16, 17, 23).
The ERK, JNK (see ref. 24), and p38 pathways are activated in response to aggregation of FcɛRI in mast cells. In this report, we have characterized the regulation of these kinases and demonstrate that the TNF-α promoter is regulated by the JNK pathway, that the upstream regulator in the JNK pathway, MEK kinase 1 (MEKK1) is activated by aggregation of FcɛRI (24), and that an inhibitory mutant JNK inhibits FcɛRI-mediated TNF-α promoter stimulation of luciferase reporter gene expression. Furthermore, FcɛRI regulation of this pathway is inhibited by wortmannin, indicating that phosphatidylinositol 3-kinase (PI3-K) activity is required for receptor stimulation of JNKs and TNF-α synthesis in response to IgE.
MATERIALS AND METHODS
Cell Line and Reagents.
The MC/9 murine mast cell clone was obtained from the American Type Culture Collection. The cells were maintained by passage in Dulbecco’s modified Eagle’s medium (DMEM, GIBCO/BRL) supplemented with 5 × 10−5 M 2-mercaptoethanol (GIBCO/BRL), 10% fetal bovine serum (Summit Biotechnology, Ft. Collins, CO), and 5% conditioned medium (rat growth factor obtained from Collaborative Biomedical Products, Bedford, MA). Mouse monoclonal IgE specific for ovalbumin (anti-OVA IgE) was prepared from the hybridoma cell line generated as described (25). Bovine myelin basic protein was obtained from Upstate Biotechnology (Lake Placid, NY). Goat affinity-purified polyclonal antibody to ERK2 (C-14, purified by binding to amino acids 345–358) was purchased from Santa Cruz Biotechnology. Ovalbumin (OVA, grade V) was obtained from Sigma. Recombinant protein G-Sepharose 4B was purchased from Zymed Laboratories (San Francisco, CA). Wortmannin was obtained from Calbiochem and stored as a 10 mM stock in dimethyl sulfoxide (DMSO). MEK inhibitor, PD 098059, was kindly provided by David Dudley (Warner Lambert Company, Ann Arbor, MI) (26, 27). Recombinant mouse TNF-α, purified rat anti-mouse TNF-α monoclonal antibody (ELISA capture), and biotinylated rabbit anti-mouse TNF-α polyclonal antibody (ELISA detection) were purchased from PharMingen. DEAE-dextran was obtained from Pharmacia Biotech.
Plasmids.
The pXP1 plasmid containing the full-length human TNF-α promoter controlling luciferase gene expression, designated pTNF(-1311)Luc, was kindly provided by James S. Economou (Division of Surgical Oncology, University of California at Los Angeles School of Medicine) (28). The NFκB-luciferase reporter plasmid was provided by Richard Flavell (Yale University, New Haven, CT) and dominant negative IκB inhibitory mutant cloned into pCMV4 (ΔN1) was provided by Lucy Ghoda (University of Colorado, Denver).
Passive Sensitization and Stimulation of MC/9 Cells.
MC/9 cells (5 × 106 per ml) were incubated with 500 ng/ml anti-OVA IgE for 2 h, then washed extensively. The cells were incubated with fresh medium for an additional 2 h. OVA dissolved in PBS was added for the stimulation, and PBS was used as a control vehicle.
Kinase Assay of JNK.
Glutathione S-transferase (GST)-c-Jun-(1–79) fusion protein was prepared as described previously and kinase activity was measured accordingly (21).
Kinase Assay of p38 MAP Kinase.
To immunoprecipitate p38 kinase, 3 × 106 cells were lysed by vigorous mixing in 0.4 ml of extraction buffer [1% Triton X-100/10 mM Tris⋅HCl, pH 7.4/5 mM EDTA/50 mM NaCl/50 mM NaF/0.1% bovine serum albumin (BSA)/20 μg/ml aprotinin/1 mM phenylmethylsulfonyl fluoride (PMSF)/2 mM Na3VO4]. The lysates were incubated with rabbit antiserum raised against the COOH-terminal peptide sequence of p38 (1:400 dilution) for 2 h at 4°C. Recombinant protein G-Sepharose 4B was added to the lysate and incubated for an additional 1 h at 4°C. The immunoprecipitates were washed once with extraction buffer, twice with PAN buffer [10 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (Pipes), pH 7.0/100 mM NaCl, 20 μg/ml aprotinin]. For the in vitro kinase assay, the immunoprecipitates were suspended in 25 μl of assay buffer (25 mM Hepes, pH 7.4/25 mM β-glycerophosphate/25 mM NaCl/2 mM DTT/0.1 mM Na3VO4) containing a recombinant NH2-terminal fragment of ATF-2 (20–50 ng) as a substrate and 5 μCi of [γ-32P]ATP and incubated for 15 min at 30°C. The kinase reaction was terminated by the addition of 4× protein loading buffer, and the mixture was boiled for 5 min and separated by electrophoresis on an SDS/12% polyacrylamide gel. The gel was fixed with 5% acetic acid and 10% methanol solution, dried, and subjected to autoradiography. The kinase activity was quantified with a PhosphorImager (Molecular Dynamics).
Kinase Assay of ERK2.
In vitro kinase assay of ERK2 was carried out as described previously (29).
Enzyme-Linked Immunosorbent Assay (ELISA) for TNF-α.
Purified rat anti-mouse TNF-α monoclonal antibody was diluted to 2 μg/ml in coating solution (0.1 M NaHCO3, pH 8.2) and 50 μl was added to wells of an ELISA plate (Dynatech). After overnight incubation at 4°C, wells were washed twice with washing solution (0.05% Tween-20/PBS) and blocked with PBS containing 10% fetal calf serum (10% FCS/PBS) at room temperature for 2 h. After washing twice, standards (30 pg/ml to 2 ng/ml recombinant mouse TNF-α) and samples were added at 100 μl per well and incubated overnight at 4°C. After washing four times, biotinylated rabbit anti-mouse TNF-α polyclonal antibody (1 μg/ml) was added to wells and incubated at room temperature for 45 min and wells were washed six times. Avidin-peroxidase (2 μg/ml) was added to wells and incubated at room temperature for 30 min, and wells were washed eight times. ABTS [2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid] substrate (30 mg/ml of 0.1 M citric acid, pH 4.35) and 0.03% H2O2 were added at 100 μl per well and the color reaction was allowed to develop at room temperature for 30 min. Absorbance of the plate was read at 410 nm and the results were analyzed by Microplate Manager (Bio-Rad).
Transfection.
Plasmids were transfected into MC/9 cells by using the DEAE-dextran method (30). Kinase or luciferase assays were generally performed 24 h after the transfection.
Luciferase Assay.
Cell pellets were lysed in 200 μl of a buffer containing 25 mM Tris⋅HCl (pH 7.8), 2 mM trans-1,2-cyclohexanediamine-N,N,N′,N′-tetraacetic acid (CDTA), 2 mM DTT, 10% glycerol, and 1% Triton X-100. The lysate was mixed with the Luciferase Assay Substrate containing beetle luciferin as a substrate (Promega), and chemiluminescence was measured for 30 sec as relative light units, using a luminometer (Monolight 2010, Analytical Luminescence Laboratory, San Diego). Relative light units were correlated with sample protein.
Statistical Analysis.
Student’s t test or Welch’s t test was used for the statistical analysis.
RESULTS
TNF-α Synthesis in Response to Antigen in MC/9 Mast Cells.
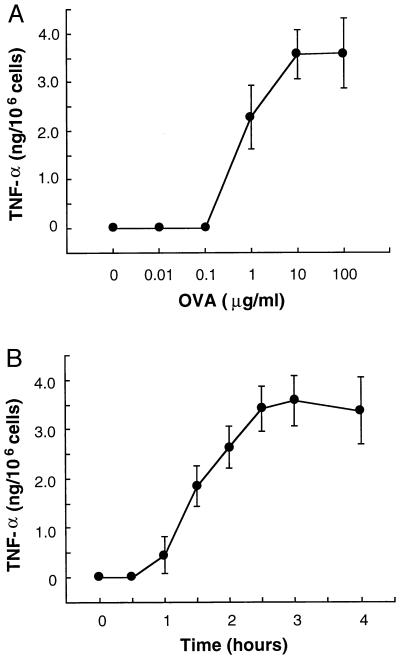
MC/9 cells previously sensitized with anti-OVA IgE were challenged in the presence or absence of OVA. Culture supernatants were harvested, and secreted TNF-α was measured by ELISA. OVA stimulated TNF-α production, in a concentration-dependent manner (Fig. 1A), which peaked at 2.5–3 h after addition of antigen (Fig. 1B). Cycloheximide (1 μg/ml) and actinomycin D (1 μg/ml) added prior to OVA challenge completely blocked the synthesis of TNF-α (data not shown), indicating that the release of TNF-α from MC/9 mast cells requires both transcription and translation. Thus, MC/9 cells are an excellent mast cell model for IgE-mediated TNF-α production and secretion.
Figure 1.
TNF-α is generated by antigen in passively sensitized MC/9 cells. (A) MC/9 cells were incubated with 500 ng/ml anti-OVA IgE for 2 h. After washing, 1 × 106 sensitized MC/9 cells were incubated in the presence of PBS (0) or 10 ng/ml to 100 μg/ml OVA for 3 h. TNF-α in the medium was measured by ELISA. OVA at concentrations of 1–100 μg/ml induced TNF-α production. TNF-α production reached maximal levels with OVA at 10 μg/ml (points represent mean ± SD, n = 6). (B) MC/9 cells sensitized with anti-OVA IgE (1 × 106 per ml) were incubated in the presence of PBS (0 h) or 10 μg/ml OVA for 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, or 4.0 h. TNF-α was detected 1 h after addition of OVA and reached maximal levels at 2.5–3.0 h (mean ± SD, n = 6).
Sequential Protein Kinase Pathways Regulated by FcɛRI Aggregation.
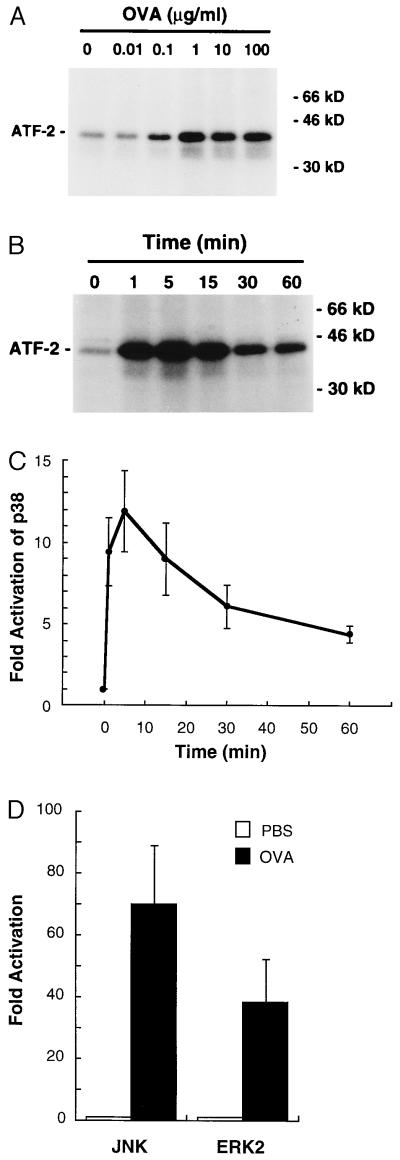
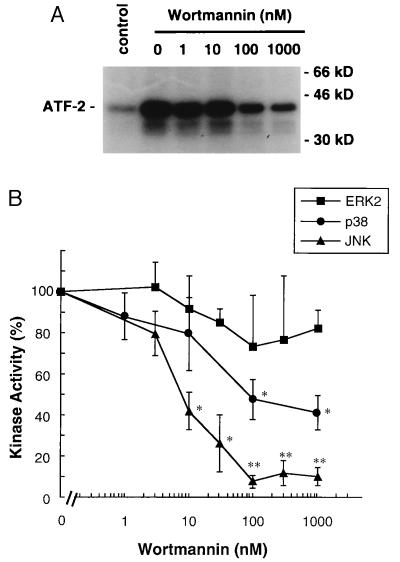
Binding of OVA to sensitized MC/9 cells results in the aggregation and activation of the FcɛRI (24). The FcɛRI activates the Src family kinases Lyn and Fyn, Syk, and the TEC family member Bruton’s tyrosine kinase (BTK) (31–34). Activation of these kinases is rapidly converted to the stimulation of phospholipase C-γ, PI3-kinase, Ras, and a number of sequential protein kinase pathways involving members of the MAP kinase family. Fig. 2 shows that ERK2, JNK, and p38 activities are all activated by aggregation of the FcɛRI receptor. However, the regulation of each pathway by the FcɛRI is different as characterized below. Using wortmannin for inhibition of PI3-K activity, it is observed that ERK activation is insensitive, JNK activation is significantly inhibited, and p38 activation is only partially inhibited by wortmannin; JNK activation is inhibited nearly 90% in the presence of 100 nM wortmannin, whereas p38 activation is inhibited by only 50–60% at 100 nM to 1 μM wortmannin (Fig. 3). The wortmannin inhibition profiles demonstrate that PI3-K activation in MC/9 cells is required for JNK activation, contributes to p38 activation, and is independent of ERK activation. The molecular basis for PI3-K involvement in the regulation of these pathways is presently unclear; it is likely that the phosphorylated lipid products of the PI3-K reaction are involved in the cellular localization of regulatory components of these pathways (35).
Figure 2.
p38 MAP kinase, JNK, and ERK2 are activated by antigen in passively sensitized MC/9 cells. (A) MC/9 cells sensitized with anti-OVA IgE were incubated in the presence of PBS (0) or OVA at 10 ng/ml to 100 μg/ml for 5 min. p38 activities in the cells were measured as described in the text. A representative autoradiograph from two independent experiments is shown. (B and C) MC/9 cells sensitized with anti-OVA IgE were incubated in the presence of PBS (0 min) or 10 μg/ml OVA for 1, 5, 15, 30, or 60 min and p38 activities in the cells were measured. A representative autoradiograph from three independent experiments (B) and the fold increase in p38 activity (mean ± SD, n = 3) (C) are shown. (D) MC/9 cells sensitized with anti-OVA IgE were incubated in the presence of PBS or 10 μg/ml OVA for 5 min (ERK2) or 15 min (JNK). Fold increases in kinase activities (mean ± SD, n = 4) are shown.
Figure 3.
Effect of wortmannin on FcɛRI-mediated activation of p38 MAP kinase, JNK, and ERK2 in MC/9 cells. (A) MC/9 cells sensitized with anti-OVA IgE (3 × 106 per ml) were incubated with 0.01% DMSO (control and 0 nM) or 1 nM to 1 μM wortmannin for 15 min. The cells were then incubated with 10 μg/ml OVA or PBS (control) for 5 min, and p38 activities in the cells were measured. A representative autoradiograph from four independent experiments is shown. (B) The effects of wortmannin on FcɛRI-mediated p38 MAP kinase activation were compared with the effects on JNK and ERK2 (mean ± SD, n = 4). The data are expressed as the percentage of kinase activities detected in the presence of 10 μg/ml OVA and 0.01% DMSO. ∗, P < 0.05; ∗∗, P < 0.01).
TNF-α Synthesis in Response to OVA Is Inhibited by Wortmannin.
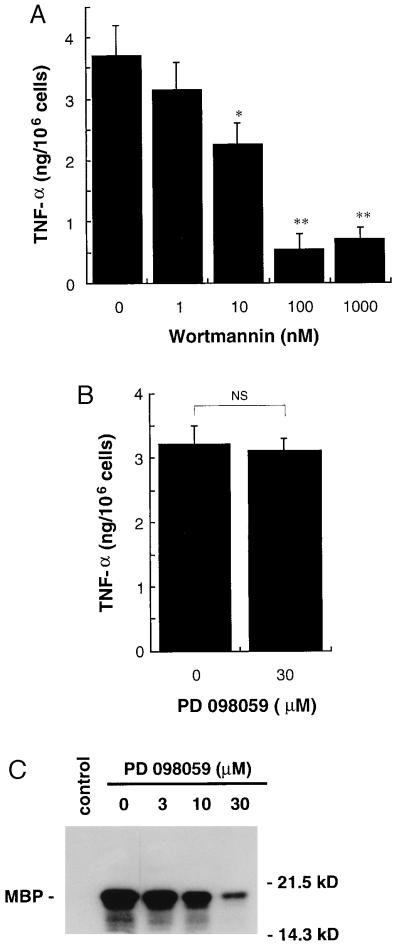
At the same dose–response relationship as inhibition of JNK activation, the treatment of MC/9 cells with wortmannin inhibited TNF-α production in response to OVA (Fig. 4A). At 100 nM wortmannin TNF-α production was inhibited nearly 90%. In contrast, the MEK inhibitor, PD 098059, had no effect on TNF-α production in response to FcɛRI aggregation induced by OVA (Fig. 4B), although 30 μM PD 098059 inhibited FcɛRI-mediated ERK2 activation by greater than 80% (Fig. 4C); the findings that ERK activation in response to OVA is not inhibited by wortmannin and that significant inhibition of ERK activation does not affect TNF-α production indicate that ERKs play little role in the FcɛRI activation of TNF-α production in MC/9 mast cells. However, the findings with wortmannin suggest that the JNKs and possibly p38 play a role in TNF-α production in response to FcɛRI aggregation.
Figure 4.
Effects of wortmannin or MEK inhibitor, PD 098059, on FcɛRI-mediated TNF-α production in MC/9 cells. (A and B) MC/9 cells sensitized with anti-OVA IgE (1 × 106 per ml) were incubated with 1 nM to 1 μM wortmannin for 15 min (A) or 30 μM PD 098059 for 1 h (B). DMSO at 0.01% or 0.1% was used as a control vehicle. The cells were then incubated with 10 μg/ml OVA for 3 h. TNF-α in the medium was measured by ELISA. Wortmannin at 1 nM to 1 μM inhibited antigen-induced TNF-α production in a dose-dependent manner (mean ± SD, n = 4; ∗∗, P < 0.01). At 30 μM PD 098059 did not affect TNF-α production (mean ± SD, n = 4; NS, not significant). (C) MC/9 cells sensitized with anti-OVA IgE (3 × 106 per ml) were incubated with 3–30 μM PD 098059 or 0.1% DMSO (control and 0 nM) for 1 h. The cells were then incubated with 10 μg/ml OVA or PBS (control) for 5 min. ERK2 activities were measured as described in the text. MBP, myelin basic protein. A representative autoradiograph from three independent experiments is shown.
Wortmannin Inhibits Activation of Transcription Regulated by the TNF-α Promoter.
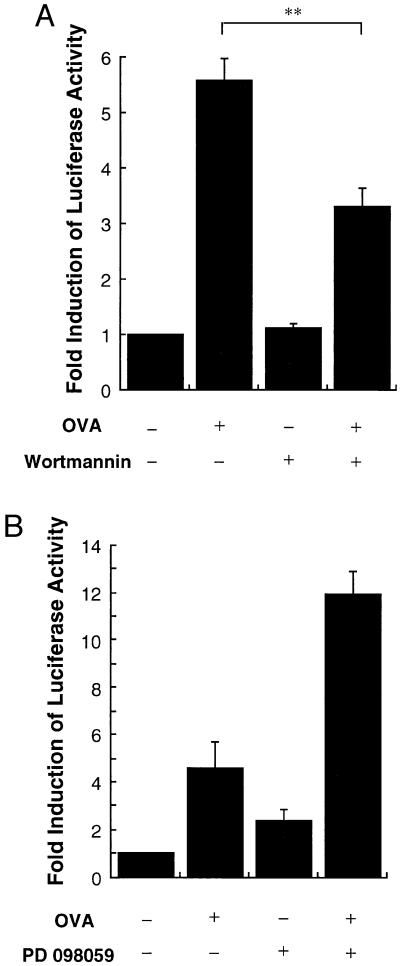
The function of p38 in the regulation of TNF-α production has been proposed to be at the level of translation (10). To test the role of these signal pathways on TNF-α gene transcription, a luciferase reporter construct under the control of the TNF-α promoter was used (28). The passively sensitized MC/9 cells, transiently transfected with the reporter plasmid pTNF(-1311)Luc, which has the luciferase gene regulated by the TNF-α promoter, responded to OVA and FcɛRI aggregation with a 5- to 6-fold increase in luciferase expression (Fig. 5). Wortmannin (100 nM) inhibited this response by 40%; in contrast, the MEK inhibitor PD 098059 actually enhanced the expression of luciferase in response to OVA as well as the basal expression without stimulation. Wortmannin did not affect the basal expression of luciferase. Thus, wortmannin inhibited the transcription from the TNF-α gene in response to OVA-induced FcɛRI aggregation. The partial inhibition of TNF-α promoter-driven luciferase expression indicated that PI3-K-regulated responses were involved in the control of TNF-α gene expression.
Figure 5.
Effects of wortmannin or PD 098059 on TNF-α promoter activity stimulated by FcɛRI aggregation. (A and B) pTNF(-1311)Luc (4 μg) was transfected into MC/9 cells. MC/9 cells were passively sensitized with anti-OVA IgE after the transfection and were incubated for additional 15 h with 10 μg/ml OVA (OVA +) or PBS (OVA −) in the presence of 0.01% DMSO (Wortmannin −) or 100 nM wortmannin (Wortmannin +) (A). Similarly, the cells were incubated with 10 μg/ml OVA (OVA +) or PBS (OVA −) in the presence of 0.1% DMSO (PD 098059 −) or 30 μM PD 098059 (PD 098059 +) (B). Luciferase activities were measured as relative light units (RLU) and standardized by control RLU (OVA −) (mean ± SD, n = 4; ∗∗, P < 0.01)
Expression of Activated MEK Kinase 1 Stimulates TNF-α Promoter-Driven Transcription.
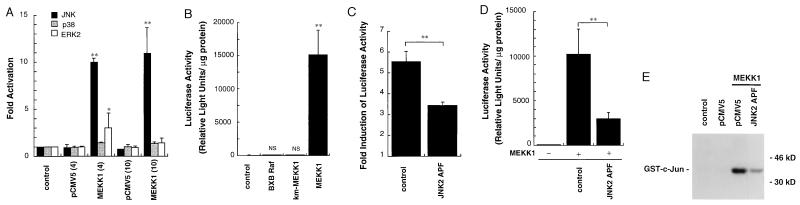
Aggregation of the FcɛRI stimulates MEKK1 activity in MC/9 cells (24). Expression of an activated form of MEKK1 (21) strongly activates JNK with little ERK and p38 activation (Fig. 6A). Activated MEKK1 expression in MC/9 cells stimulated a 1000-fold increase in luciferase activity, resulting from activation of the TNF-α promoter (Fig. 6B), demonstrating that activated MEKK1 is capable of driving TNF-α gene transcription. Activated Raf-1 (BXB Raf) or inactive MEKK1 (km-MEKK1) expression did not affect the activity of the TNF-α promoter (Fig. 6B), demonstrating that the kinase activity of MEKK1 was required for stimulation of the TNF-α promoter. An inhibitory JNK2 mutant (JNK2 APF) partially inhibited OVA- (Fig. 6C) and MEKK1-stimulated (Fig. 6D) TNF-α promoter activity (40% inhibition and 70% inhibition, respectively). Expression of JNK2 APF also partially inhibited JNK activity in response to expression of activated MEKK1 (Fig. 6E). Cumulatively, the data indicate that MEKK1 and JNK activation in response to FcɛRI aggregation contributes to regulation of TNF-α gene transcription. The partial inhibition of both JNK activation and TNF-α promoter activity by JNK 2APF is because it is a competitive inhibitory mutant; strong dominant negative mutants for JNK or its upstream regulator JNK kinase have not been developed, and the kinase-inactive mutants and phosphorylation site mutants behave as more modest competitive inhibitory mutants.
Figure 6.
Effect of MEKK1 overexpression on JNK, p38, ERK2 activity, and TNF-α promoter activity. (A) pCMV5ΔMEKK1 [4 or 10 μg, MEKK1(4) or MEKK1(10)] or an equivalent amount of pCMV5 empty plasmid [pCMV5(4) or pCMV5(10)] was transfected into MC/9 cells. Cells treated with only DEAE-dextran were used as control (control). JNK, p38, and ERK2 activities were measured at 24 h after the transfection. Kinase activities were standardized by control activities and expressed as fold activation (mean ± SD, n = 3; ∗, P < 0.05; ∗∗, P < 0.01). (B) pCMV5ΔMEKK1 (MEKK1, 2 μg), pCMV5 empty plasmid (2 μg; control), pCMV5BXBRaf (BXB Raf, 2 μg), or pCMV5km-MEKK1 (km-MEKK1, 2 μg) was transfected into MC/9 cells with pTNF(-1311)Luc (2 μg). Luciferase activities were measured as relative light units 24 h after the transfection. (C) An inhibitory JNK2 mutant expression plasmid (JNK2 APF, 4 μg) or equivalent amount of control plasmid (control), and pTNF(-1311)Luc (2 μg) were transfected into MC/9 cells. MC/9 cells were passively sensitized with anti-OVA IgE after the transfection and were incubated for an additional 15 h with 10 μg/ml OVA or PBS. Luciferase activities were expressed as fold induction by addition of OVA. (D) An inhibitory JNK2 mutant expression plasmid (JNK2 APF, 4 μg) or equivalent amount of control plasmid (control), pCMV5ΔMEKK1 (MEKK1, 2 μg) or control plasmid, and pTNF(-1311)Luc (2 μg) were transfected into MC/9 cells. Luciferase activities were measured 24 h after the transfection. (E) An inhibitory JNK2 mutant expression plasmid (JNK2 APF, 4 μg), or equivalent amount of control plasmid (pCMV5), and pCMV5ΔMEKK1 (MEKK1, 2 μg) were transfected into MC/9 cells. Only control plasmid (6 μg) was also transfected independently (pCMV5). Cells treated with only DEAE-dextran were also used as control (control). JNK activities were assayed 24 h after the transfection. A representative autoradiograph from two independent experiments is shown.
Inhibition of NFκB Activation Does Not Affect TNF-α Promoter Activation.
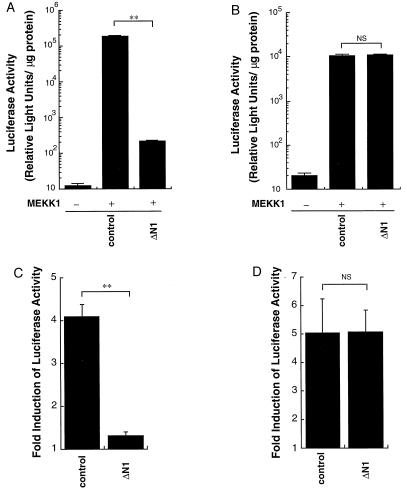
MEKK1 has been shown to stimulate NFκB activity (23), and NFκB has been proposed to be a regulator of TNF-α gene transcription (16, 17). To determine if NFκB plays a role in FcɛRI and MEKK1 regulation of TNF-α promoter regulation, a dominant negative IκB inhibitory mutant (ΔN1) was used. The IκB inhibitory mutant is NH2-terminally truncated, removing the phosphorylation sites that induce its degradation (36–38). Expression of the ΔN1 mutant significantly inhibited both MEKK1- and OVA-induced NFκB activation (Fig. 7 A and C). However, expression of ΔN1 did not affect MEKK1- or OVA-induced stimulation of the promoter-driven luciferase expression (Fig. 7 B and D). The induction of luciferase by OVA was similar between pNFκB Luc-transfected cells and pTNF(-1311)Luc-transfected cells (Fig. 7 C and D), indicating that the promoters were similarly responsive to receptor stimulation. The results indicate that MEKK1- and IgE-regulated pathways, independent of NFκB, are responsible for control of TNF-α gene expression.
Figure 7.
Effects of inhibitory IκB mutant on activation of TNF-α gene promoter in MC/9 cells. (A and B) An inhibitory IκB mutant ΔN1 expression plasmid (ΔN1, 4 μg) or equivalent amount of control plasmid (control), pCMV5ΔMEKK1 (2 μg) (MEKK1 +) or control plasmid (MEKK1 −), and pNFκB Luc (2 μg) (A) or pTNF(-1311)Luc (2 μg) (B) was transfected into MC/9 cells. Luciferase activities were measured 24 h after the transfection (∗∗, P < 0.01; NS, not significant). (C and D) An inhibitory IκB mutant ΔN1 expression plasmid (ΔN1, 4 μg) or equivalent amount of control plasmid (control), and pNFκB Luc (2 μg) (C) or pTNF(-1311)Luc (2 μg) (D) was transfected into MC/9 cells. MC/9 cells were passively sensitized with anti-OVA IgE after the transfection and were incubated for an additional 15 h with 10 μg/ml OVA or PBS. Luciferase activities were expressed as fold induction by addition of OVA (∗∗, P < 0.01; NS, not significant).
p38 Inhibitor Does Not Affect TNF-α Promoter Activation and TNF-α Production.
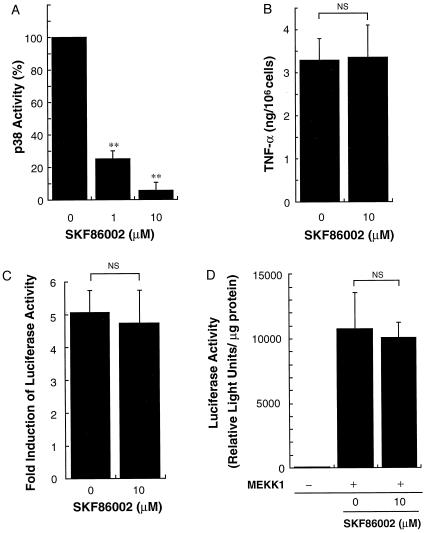
Addition of 10 μM SKF 86002 inhibited OVA-induced p38 activity by more than 90% (Fig. 8A). However, 10 μM SKF 86002 did not affect FcɛRI-mediated TNF-α production (Fig. 8B). Similarly, 10 μM SKF 86002 did not affect TNF-α promoter activity stimulated by FcɛRI aggregation or MEKK1 expression (Fig. 8 C and D). These results suggest that significant p38 activity is not required or involved in regulation of the TNF-α promoter.
Figure 8.
Effect of p38 inhibitor SKF86002 on p38 activities, TNF-α production, and TNF-α promoter activity. (A) MC/9 cells sensitized with anti-OVA IgE were incubated in the presence of 10 μg/ml OVA for 5 min and p38 activities were measured. SKF 86002 at 1–10 μM significantly inhibited p38 activity. The data are expressed as the percentage of kinase activity detected in the presence of 10 μg/ml OVA and 0.1% DMSO (mean ± SD, n = 4; ∗∗, P < 0.01). (B) MC/9 cells sensitized with anti-OVA IgE (1 × 106 per ml) were incubated with 0.1% DMSO or 10 μM SKF 86002 for 2 h. The cells were then incubated with 10 μg/ml OVA for 3 h. SKF 86002 at 10 μM did not affect TNF-α production (mean ± SD, n = 4; NS, not significant). (C) pTNF(-1311)Luc (4 μg) was transfected into MC/9 cells. MC/9 cells were passively sensitized with anti-OVA IgE after the transfection and were incubated for an additional 15 h with 10 μg/ml OVA or PBS in the presence of 0.1% DMSO (0) or 10 μM SKF 86002. Luciferase activities were measured as relative light units (RLU) and standardized by control RLU (PBS) (mean ± SD, n = 4; NS, not significant). (D) pCMV5ΔMEKK1 (MEKK1, 2 μg) or pCMV5 empty plasmid (pCMV5, 2 μg) was transfected into MC/9 cells with pTNF(-1311)Luc (2 μg) in the presence of 0.1% DMSO (0) or 10 μM SKF 86002. Luciferase activities were measured as relative light units 24 h after the transfection (mean ± SD, n = 4; NS, not significant).
DISCUSSION
The TNF-α promoter has been defined to encode a κ3 response element that binds NFATp and a calcium and cyclic AMP response element (CRE) that binds ATF-2 and Jun proteins (13, 20). In addition, there are AP-1 and AP-2 sites in the TNF-α promoter that have been shown to bind bZIP proteins that include Fos and Jun (14, 18). In T cells, it was recently shown that the CRE sites, but not the AP-1 or AP-2 sites, in the TNF-α promoter are important for T cell receptor stimulation of TNF-α gene transcription (13). The CRE site augmented the k3 site in inducing TNF-α gene expression (13).
c-Jun and ATF-2 are substrates for the JNKs (39–42), and ATF-2 has also been shown to be a substrate for the p38 kinase (43). In our studies, we have defined a stimulatory role for the MEKK1/JNKK/JNK sequential protein kinase pathway in the regulation of TNF-α gene expression in mast cells. Expression of activated MEKK1 strongly stimulates JNK activity, weakly activates ERK2 activity, and gives an extremely robust activation of TNF-α promoter-driven luciferase expression. The MEK inhibitor PD 098059 inhibited OVA-induced ERK2 activation but did not affect TNF-α production and augmented the activity of the TNF-α promoter. These results indicate that ERK2 is not involved in TNF-α production in mast cells. NFκB activation similarly did not play a significant role in the regulation of TNF-α gene expression. Furthermore, a kinase-inactive inhibitory mutant of JNK2 (JNK2 APF) significantly blunted both MEKK1- and OVA-IgE mediated stimulation of TNF-α gene expression. These results demonstrate that MEKK1 stimulation by the FcɛRI activates a JNK pathway that contributes to the activation of the TNF-α promoter. Thus, a model emerges from our results in mast cells and the studies of others in T cells (12, 13) that the activation of the MEKK1 pathway and calcineurin regulation of NFATp contribute to the stimulation of TNF-α gene expression. The involvement of the MEKK1 pathway in regulating the TNF-α promoter defines a mechanism for the previously described CRE augmentation of κ3 regulation of TNF-α gene expression.
At present we cannot unequivocally define the role of p38 in the MEKK1 regulation of TNF-α gene expression. The p38 kinase activity is weakly activated by MEKK1. Our studies with the p38 inhibitor SKF 86002 (10) demonstrated no effect on TNF-α gene expression, suggesting that p38 does not play a significant role in the regulation of the TNF-α promoter. SKF 86002 did not affect OVA-induced TNF-α production in MC/9 cells, although it did inhibit p38 activity. The work of others has suggested that p38 primarily functions to regulate cytokine production at a post-transcriptional level involving translation of the cytokine (10). Taken together, the very small activation of p38 following activated MEKK1 expression and the studies with the inhibitor suggest that JNKs and not p38 kinase primarily mediate MEKK1 and FcɛRI stimulation of TNF-α gene expression. However, further genetic and biochemical studies are warranted to address this issue.
The inhibitory effects of wortmannin on TNF-α production and gene expression suggest a role for PI3-K in controlling TNF-α synthesis in mast cells. FcɛRI activation is well defined in its ability to activate PI3-K (44), and a role for PI3-K is emerging for its regulation of signal transduction pathways (24, 44). Thus, the activation of PI3-K is important for both the synthesis and secretion of cytokines such as TNF-α. Cumulatively, it appears that calcineurin and NFATp, the MEKK1/JNKK/JNK pathway, the p38 kinase, and PI3-K are critical for the synthesis and secretion of cytokines such as TNF-α in response to the activation of FcɛRI.
For the mast cell, there is a rapid increase in cytokine production following activation by allergen (2–4). It is believed that cytokines, including TNF-α, play major roles in triggering and sustaining the allergic inflammatory response. Our findings define the MEKK1 pathway and PI3-K as important therapeutic targets in addition to calcineurin and NFATp for controlling allergic inflammatory responses.
Acknowledgments
We thank Dr. James S. Economou and Dr. Kristina L. Rhoades for providing pTNF(-1311)Luc and Dr. David Dudley for providing PD 098059. The assistance of Dr. Naoki Sakata and Dr. Hideki Kawasome is greatly appreciated. This work was supported in part by grants AI HL-36577 (E.W.G.) and DK-37871 (G.L.J.) from the National Institutes of Health.
ABBREVIATIONS
- FcɛRI
high-affinity Fc receptor for IgE
- TNF-α
tumor necrosis factor α
- MAP kinase
mitogen-activated protein kinase
- ERK
extracellular signal-regulated kinase
- JNK
c-Jun amino-terminal kinase
- MEKK1
MEK kinase 1
- PI3-K
phosphatidylinositol 3-kinase
- OVA
ovalbumin
- PMSF
phenylmethylsulfonyl fluoride
- DMSO
dimethyl sulfoxide
- CRE
calcium and cyclic AMP response element
References
- 1.Plaut M, Pierce J H, Watson C J, Hanley-Hyde J, Nordan R P, Paul W E. Nature (London) 1989;339:64–67. doi: 10.1038/339064a0. [DOI] [PubMed] [Google Scholar]
- 2.Gordon J R, Galli S J. Nature (London) 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 3.Gordon J R, Galli S J. J Exp Med. 1991;174:103–107. doi: 10.1084/jem.174.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wodnar-Filipowicz A, Heusser C H, Monori C. Nature (London) 1989;339:150–152. doi: 10.1038/339150a0. [DOI] [PubMed] [Google Scholar]
- 5.Messadi D V, Pober J S, Fiers W, Gimbrone M A, Murphy G F. J Immunol. 1987;139:1557–1562. [PubMed] [Google Scholar]
- 6.Walsh L J, Trinchieri G, Waldorf H A, Whitaker D, Murphy G F. Proc Natl Acad Sci USA. 1991;88:4220–4224. doi: 10.1073/pnas.88.10.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hultsch T, Albers M W, Schreiber S L, Hohman R J. Proc Natl Acad Sci USA. 1991;88:6229–6233. doi: 10.1073/pnas.88.14.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaye R E, Fruman D A, Bierer B E, Albers M W, Zydowsky L D, Ho S I, Jin Y, Castells M C, Schreiber S L, Walsh C T, Burakoff S J, Austen K F, Katz H R. Proc Natl Acad Sci USA. 1992;89:8542–8546. doi: 10.1073/pnas.89.18.8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keegan A D, Paul W E. Immunol Today. 1992;13:63–68. doi: 10.1016/0167-5699(92)90136-U. [DOI] [PubMed] [Google Scholar]
- 10.Lee J C, Laydon J T, McDonnell P C, Gallagher T F, Kumar S, Green D, McNulty D, Blumenthal M J, Heys J R, Landvatter S W, Strickler J E, McLaughlin M M, Siemens I R, Fisher S M, Livi G P, White J R, Adams J L, Young P R. Nature (London) 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 11.McCaffrey P G, Goldfeld A E, Rao A. J Biol Chem. 1994;269:30445–30450. [PubMed] [Google Scholar]
- 12.Goldfeld A E, Tsai E, Kincaid R, Belshaw P J, Schreiber S L, Strominger J L, Rao A. J Exp Med. 1994;180:763–768. doi: 10.1084/jem.180.2.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai E Y, Jain J, Pesavento P A, Rao A, Goldfeld A E. Mol Cell Biol. 1996;16:459–467. doi: 10.1128/mcb.16.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rao A. Immunol Today. 1994;15:274–281. doi: 10.1016/0167-5699(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 15.Rao A. J Leukocyte Biol. 1995;57:536–542. doi: 10.1002/jlb.57.4.536. [DOI] [PubMed] [Google Scholar]
- 16.Shakhov A N, Collart M A, Vassalli P, Nedospasov S A, Jongeneel C V. J Exp Med. 1990;171:35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drouet C, Shakhov A N, Jongeneel C V. J Immunol. 1991;147:1694–1700. [PubMed] [Google Scholar]
- 18.Economou J S, Rhoades K, Essner R, McBride W H, Gasson J C, Morton D L. J Exp Med. 1989;170:321–326. doi: 10.1084/jem.170.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krämer B, Wiegmann K, Kronke M. J Biol Chem. 1995;270:6577–6583. doi: 10.1074/jbc.270.12.6577. [DOI] [PubMed] [Google Scholar]
- 20.Newell C L, Deisseroth A B, Lopez-Berestein G. J Leukocyte Biol. 1994;56:27–35. doi: 10.1002/jlb.56.1.27. [DOI] [PubMed] [Google Scholar]
- 21.Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis R J, Johnson G L, Karin M. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 22.Yan M, Dai T, Deak J C, Kyriakis J M, Zon L I, Woodgett J R, Templeton D J. Nature (London) 1994;372:798–800. doi: 10.1038/372798a0. [DOI] [PubMed] [Google Scholar]
- 23.Hirano M, Osada S, Aoki T, Hirai S, Hosaka M, Inoue J, Ohno S. J Biol Chem. 1996;271:13234–13238. doi: 10.1074/jbc.271.22.13234. [DOI] [PubMed] [Google Scholar]
- 24.Ishizuka T, Oshiba A, Sakata N, Terada N, Johnson G L, Gelfand E W. J Biol Chem. 1996;271:12762–12766. doi: 10.1074/jbc.271.22.12762. [DOI] [PubMed] [Google Scholar]
- 25.Oshiba A, Hamelmann E, Takeda K, Bradley K L, Loader J E, Larsen G L, Gelfand E W. J Clin Invest. 1996;97:1398–1408. doi: 10.1172/JCI118560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. J Biol Chem. 1995;46:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 27.Pang L, Sawada T, Decker S J, Saltiel A R. J Biol Chem. 1995;270:13585–13588. doi: 10.1074/jbc.270.23.13585. [DOI] [PubMed] [Google Scholar]
- 28.Rhoades K L, Golub S H, Economou J S. J Biol Chem. 1992;267:22102–22107. [PubMed] [Google Scholar]
- 29.Cook S J, McCormick F. Science. 1993;262:1069–1072. doi: 10.1126/science.7694367. [DOI] [PubMed] [Google Scholar]
- 30.Heike T, Miyatake S, Yoshida M, Arai K, Arai N. EMBO J. 1989;8:1411–1417. doi: 10.1002/j.1460-2075.1989.tb03522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eiseman E, Bolen J B. Nature (London) 1992;355:78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- 32.Hutchcroft J E, Geahlen R L, Deanin G G, Oliver J M. Proc Natl Acad Sci USA. 1992;89:9107–9111. doi: 10.1073/pnas.89.19.9107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiue L, Green J, Green O M, Karas J L, Morgenstern J P, Ram M K, Taylor M K, Zoller M J, Zydowsky L D, Bolen J B, Brugge J S. Mol Cell Biol. 1995;15:272–281. doi: 10.1128/mcb.15.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawakami Y, Yao L, Miura T, Tsukada S, Witte O N, Kawakami T. Mol Cell Biol. 1994;14:5108–5113. doi: 10.1128/mcb.14.8.5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klippel A, Reinhard C, Kavanaugh W M, Apell G, Escobedo M-A, Williams L T. Mol Cell Biol. 1996;16:4117–4127. doi: 10.1128/mcb.16.8.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 37.Traenckner E B-M, Pahl H L, Henkel T, Schmidt K N, Wilk S, Baeuerle P A. EMBO J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brockman J A, Scherer D C, McKinsey T A, Hall S M, Qi X, Lee W Y, Ballard D W. Mol Cell Biol. 1995;15:2809–2818. doi: 10.1128/mcb.15.5.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hibi M, Lin A, Smeal T, Minden A, Karin M. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- 40.Derijard B, Hibi M, Wu I-H, Barrett T, Su B, Deng T, Karin M, Davis R J. Cell. 1994;76:1025–1038. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 41.Derijard B, Raingeaud J, Barrett T, Wu I H, Han J, Ulevitch R J, Davis R J. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 42.Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Avruch J, Woodgett J. Nature (London) 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 43.Raingeaud J, Gupta S, Rogers J S, Dickens M, Han J, Ulevitch R J, Davis R J. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 44.Yano H, Nakanishi S, Kimura K, Hanai N, Saitoh Y, Fukui Y, Nonomura Y, Matsuda Y. J Biol Chem. 1993;268:25846–25856. [PubMed] [Google Scholar]