Abstract
Secretory IgA (sIgA) plays a critical role in providing protection against infection at the mucosal surfaces. Normally, sIgA is the product of two different cell types with heavy, light, and J chains produced by the plasma cells, whereas secretory component (SC), a cleavage product of the polymeric immunoglobulin receptor (pIgR), is added during the transit of dimeric IgA through the epithelial cell layer. In the current study, by introducing a gene for the processed form of SC into a cell line that produces dimeric IgA, we have succeeded in creating a single cell that is able to produce and secrete covalently joined sIgA. To our knowledge, this is the first time it has been possible to efficiently produce large quantities of sIgA of defined specificity in mammalian cells. The sIgA made using this approach has great potential as an immunotherapeutic.
Secretory IgA (sIgA) provides the first line of immune defense at the mucosal surfaces of the gastrointestinal, respiratory, and genitourinary tracts, where more than 95% of infections are initiated. In vivo, sIgA is the product of two different cell types, the plasma cell and the epithelial cell (1, 2). Plasma cells synthesize and assemble α H chains and L chains with J chain into polymeric IgA. The polymeric IgA secreted by the plasma cell binds to the polymeric Ig receptor (pIgR) expressed on the basolateral surface of the mucosal epithelium. The IgA–pIgR complex is transcytosed to the apical surface; during transit a disulfide bond is formed between the IgA and the pIgR. At the apical surface, an unknown enzyme cleaves between the ectoplasmic domain [also known as secretory component (SC)] and the transmembrane domain, releasing the IgA–SC complex into external secretions.
Passive administration of IgA potentially could provide protection against a wide range of pathogens including bacteria and viruses such as HIV and respiratory synctial virus. Hybridoma-produced IgA antibodies applied directly to mucosal surfaces or transported into external secretions after injection into blood are protective, but have been found to be rapidly degraded (3–5). In vitro, sIgA is more resistant to proteases than serum IgA (6–7), suggesting that sIgA would be a more effective molecule for therapeutic use. However, until now, coculture systems containing hybridomas and polarized monolayers of epithelial cells (8) and in vitro mixing of purified polymeric IgA (pIgA) and SC (9) have succeeded in producing only analytical quantities of sIgA.
We now report the production of sIgA by a single mammalian cell, an approach that makes possible the production of large quantities of sIgA.
MATERIALS AND METHODS
Reagents and Cells.
Restriction endonucleases and molecular cloning enzymes were obtained from either New England Biolabs, Pharmacia, Stratagene, or Promega and used according to the manufacturers’ suggestions. [35S]α-ATP and [35S]methionine were obtained from ICN. The human pIgR gene was obtained from Charlotte S. Kaetzel (University of Kentucky, Lexington) (10). The IgA1-secreting transfectant previously described (11) was transfected with human SC to produce sIgA. Cells were cultured in Iscove’s modified Dulbecco’s medium (IMDM) containing 5% bovine calf serum (HyClone).
Construction of a Human SC Expression Vector.
A 1402-bp PCR fragment was generated by using the complete human pIgR cDNA in pBluescript as template and the primers 5′-GGGCAGAACGGTGACCATCAACTGCCCTTT-3′ and 5′-AAGGAATTCCTACTCTGCAAAAAGCCTGGGGTCCTGAATGGC-3′. The primer included a silent base change upstream of Glu589 to delete a BamHI site in the SC coding region to facilitate cloning. A stop codon, which is underlined, followed by an EcoRI site downstream of Glu589 were also included. The PCR product was cloned into TA vector (Invitrogen), and the sequence was confirmed by sequencing. The complete human SC gene was generated by a three-way ligation of an EcoRI-KpnI fragment that included the Kozak sequence, the leader sequence, and the 5′ SC sequence, and a KpnI-EcoRI PCR fragment that included the 3′ SC sequence and an EcoRI linearized pBluescript II KS(+) containing the Ig poly(A)+ signal. A 3.28-kb EcoRV-BamHI fragment containing the complete SC gene was ligated downstream of an Ig promoter in a pSV2 expression vector containing the his gene as a selection marker.
Production of Transfectants Secreting sIgA.
Approximately 6 × 106 cells expressing chimeric IgA1 were washed and resuspended in 0.9 ml of cold IMDM. Cells were incubated on ice for 10 min with 10 μg of PvuI-linearized DNA in 0.1 ml IMDM. Cells were pulsed with an electric field of 200 V and 960 μF in a gene-pulser apparatus (Bio-Rad); washed once with IMDM and resuspended in 37.5 ml IMDM containing 10% fetal calf serum, 100 μg/ml of gentamycin, and 100 units per ml of Nystatin (GIBCO/BRL); and plated in three 96-well plates at 125 μl per well. After 2 days of growth, an equal volume of medium containing 5 mM histidinol was added to the wells to select for the histidinol-resistant cells. After 3 more days, half the medium was replaced by an equal volume of selection medium. After 2 weeks, the surviving colonies were screened for sIgA secretion by ELISA.
ELISA.
Microtiter plates were incubated overnight at 4°C with 50 μl of 50 μg/ml dansylated BSA in Na2CO3 buffer, pH 9.6. The plates were washed with PBS and the residual sites were blocked with PBS (pH 6.8) containing 3% BSA. Fifty microliters of supernatants from the 96-well plates containing transfectants was transferred directly into the antibody-coated plates and incubated overnight. Plates were washed with PBS, and the bound sIgA was detected by incubation with rabbit antiserum to human SC (12) diluted 1:2,000 (vol/vol) in PBS containing 1% BSA (PBS–1% BSA). Bound rabbit antibody was detected by incubating with alkaline phosphatase-conjugated goat anti-rabbit IgG (Sigma) diluted 1:10,000 in PBS–1% BSA. All incubations were for 2 hr at room temperature with three washes with PBS after each step. Color was developed by adding 5 mg/ml of disodium p-nitrophenyl phosphate (Sigma) in diethanolamine buffer, pH 9.8.
Biosynthetic Labeling and Pulse-Chase Analysis.
About 6 × 106 cells were washed twice with cysteine-free medium and incubated at 37° in 0.6 ml of cysteine-free medium for 30 min to deplete intracellular stores of cysteine. Cells were pulsed by adding 75 μCi (1 Ci = 37 GBq) of [35S]cysteine and incubating at 37°C for 20 min. Cells were pelleted by centrifuging at 1,000 × g for 5 min, the supernatant was removed and the chase initiated by adding 6 ml of medium containing 20% bovine calf serum and 0.4 mg/ml of unlabeled cysteine. At various times after the initiation of the chase 1 ml aliquots of cells were removed and cooled to 0°C by adding to 3 ml of chilled DMEM containing 30 mM iodoacetamide. Cells were pelleted by centrifugation and lysed by boiling in 20 mM triethanolamine⋅HCl buffer, pH 8.1 (buffer A), containing 2% SDS (13) and 25 mM iodoacetamide. After cooling, 4 volumes of 20 mM of triethanolamine⋅HCl buffer, pH 8.1, containing 2.5% Triton X-100, 25 mM iodoacetamide, 150 mM NaCl, 5 mM EDTA, and 0.1% soybean trypsin inhibitor (buffer B) was added. The lysates were centrifuged at 13,000 × g for 10 min to remove cell debris and DNA. SDS was added to yield a final concentration of 2%, and the lysates were boiled and then diluted with 4 volumes of buffer B.
Protein Iodination.
Approximately 25 μg of IgA and sIgA purified from supernatants by dansyl-Sepharose affinity chromatography were iodinated using Iodogen reagent (Pierce) and 200 μCi of 125I as sodium salt (Amersham) to obtain a specific activity of 2–3 × 106 cpm/μg. After dialysis against PBS containing 10 mM KI greater than 98% of the radioactivity was precipitable with TCA. The integrity and purity of the iodinated proteins were confirmed by SDS/PAGE in 5% phosphate gels (see Fig. 4B).
Figure 4.
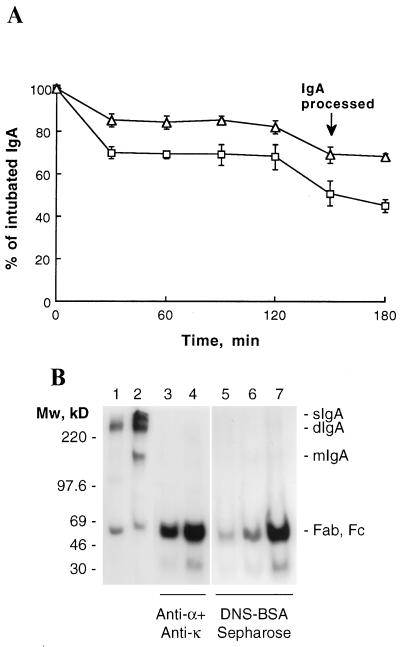
In vivo stability of sIgA. (A) 125I-labeled dIgA (squares) and sIgA (triangles) were introduced directly into the stomach of BALB/c mice by intubation. IgA remaining in the mice was determined by whole body counting. Data are expressed as mean ± SD (n = 3). (B) After 150 min, a mouse intubated with dIgA (lanes 3 and 6) and a mouse intubated with sIgA (lanes 4 and 7) were killed and the intestinal washings isolated and processed as described. IgA from the intestinal washes was immunoprecipitated with either anti-α and anti-κ antibodies (lanes 3 and 4) or with DNS-BSA-Sepharose (lanes 6 and 7). For comparison, mice injected i.v. with radiolabeled dIgA were sacrificed after 3 hr, and the antigen-specific IgA was precipitated from the intestinal washes as above (lane 5). Half the precipitated proteins were analyzed by SDS/PAGE in phosphate gels. The gels were dried and exposed to Amersham Hyperfilm-MP for 48 hr. Also shown are the iodinated dIgA (lane 1) and sIgA (lane 2) used for intubate. The prestained molecular mass protein standards (Amersham) are indicated on the left; the positions of sIgA, dIgA, mIgA, Fab, and Fc, on the right.
Intubation and Isolation of Intestinal Contents.
Nine-week-old female BALB/c mice (Taconic Farms) were lightly anesthetized by ether, and radiolabeled IgA was delivered into the stomach by intubation through polyethylene tubing attached to an 18-gauge needle on a hypodermic syringe.
After 2.5 hr the mice were killed and the intestinal contents were isolated and processed by a modified method of Elson et al. (14). Intestines from duodenum to rectum were removed and injected with 4 ml of PBS, pH 7.2, containing 0.1 mg/ml soybean trypsin inhibitor, 50 mM EDTA, and 1 mM phenylmethylsulfonyl fluoride (PMSF). The intestinal contents were squeezed out into a Petri dish on ice, homogenized using a spatula, and transferred into a microfuge tube. The homogenate was vortexed and centrifuged at 13,000 × g to separate the particulate material. The extracts were supplemented with 1 mM PMSF and 0.05% NaN3.
To immunoprecipitate IgA, an aliquot of intestinal washes containing approximately 100,000 cpm of intestinal washes were incubated on ice with an excess of anti-α and anti-κ followed by protein G Sepharose (Sigma) in PBS. Precipitates were washed three times with PBS and then counted. Electrophoresis sample buffer was added to the precipitates, boiled, and half the supernatant was analyzed by SDS/PAGE in 5% phosphate gels. To immunoprecipitate antigen-specific IgA, approximately 100,000 cpm were incubated on ice with an excess of dansylated BSA coupled to Sepharose beads (DNS-BSA-Sepharose). After washing, bound antibody were eluted by incubating the beads for 10 min on ice in 30 μl of 3 mM ɛ-dansyl-l-lysine (Sigma). Half the eluted proteins were analyzed by SDS/PAGE in 5% phosphate gels. The gels were dried and exposed to Amersham Hyperfilm-MP for 48 hr.
RESULTS AND DISCUSSION
Expression of SC in Transfectants Secreting IgA1.
Because SC is a cleavage product of the pIgR we introduced a stop codon at the site of cleavage (Fig. 1A). We reasoned that more efficient production could be achieved by providing SC instead of relying on the cell to cleave the pIgR into the appropriate product. Murine transfectomas secreting mouse–human chimeric IgA1 specific for the hapten dansyl (11) were transfected with the SC expression vector by electroporation, and stable transfectants were selected with histidinol. After 2 weeks, the surviving colonies were screened for sIgA production by ELISA using antigen-coated microtiter plates to capture the antibody and rabbit anti-human SC as the detecting antibody. Positive colonies were expanded and cultured in medium containing [35S]cysteine. SDS/PAGE analysis under reducing conditions of the sIgA precipitated from culture supernatants with DNS-BSA-Sepharose revealed bands corresponding to SC, H, L, and J chains (data not shown).
Figure 1.
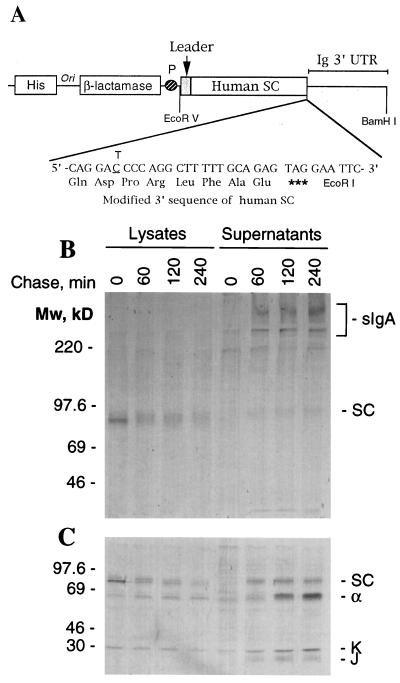
Expression of chimeric sIgA in Sp2/0 cells. (A) Schematic representation of the human SC expression vector containing genes for histidinol and ampicillin resistance as well as a 3.2-kb EcoRV-BamHI DNA fragment including a 1.82-kb human SC coding sequence with a stop codon introduced at amino acid 590, the position of normal SC processing, fused to a 1.42-kb Ig 3′ region with a poly(A) addition site (Ig 3′ untranslated region). (B) Assembly and secretion of SC. Transfectants secreting sIgA were pulsed for 5 min with a mixture of 35S-labeled methionine and cysteine and chased with a 100-fold excess of unlabeled methionine and cysteine. At the specified times, cells were rapidly cooled to 0°C and lysed by boiling in buffer containing SDS. SC and molecules covalently bound to SC were precipitated as described. The immune complexes were analyzed by SDS/PAGE in 6% Tris⋅glycine gels under nonreducing conditions. Molecular mass standards are indicated on the left. The positions of sIgA and SC are marked on the right. (C) Analysis of immunoprecipitates in 12.5% Tris⋅glycine gels under reducing conditions. The prestained molecular mass protein standards (Amersham) are indicated on the left; the positions of SC, α, κ, and J chain, on the right.
Assembly of sIgA.
Pulse-chase analysis was used to determine whether the transfectants were able to form covalently associated sIgA (11). Cells pulsed for 20 min in medium containing [35S]cysteine were chased with 100-fold excess cold cysteine, and the assembly of the labeled SC and IgA into covalently associated sIgA followed. To immunoprecipitate only IgA covalently associated with SC, proteins were first denatured by boiling cell lysates and culture supernatants in buffer containing 2% SDS (13). The SDS then was diluted to form micelles with buffer containing Triton X-100 and iodoacetamide, and SC was immunoprecipitated with rabbit anti-human SC from cell lysates and supernatants (12). The immunoprecipitates were analyzed by SDS/PAGE in the absence (Fig. 1B) or the presence (Fig. 1C) of a reducing agent. Immediately after the pulse a sharp band of SC with a molecular mass of 77 kDa was precipitated from the cellular lysate; with time, this band became diffuse, indicating glycosylation of SC as it moved along the secretory pathway (Fig. 1B). Little covalently associated sIgA was observed within the cell, although a small amount of H and L chain could be seen following reduction of the immunoprecipitates (Fig. 1C). SC was efficiently secreted with 45% present in the supernatant by 60 min and 75% secreted by 4 hr. Notably, virtually all of the SC was secreted covalently associated in sIgA, with only a minor band of free SC with a molecular mass of 80 kDa seen in the supernatant. Densitometric analysis of the secreted proteins showed approximately four α chains were present per each molecule of SC and J chain, suggesting that one molecule each of J chain and SC was present per dIgA (data not shown).
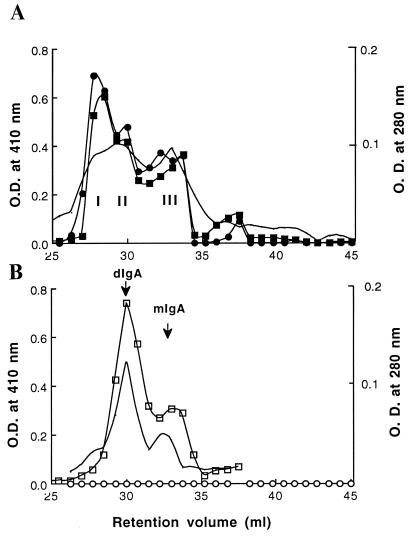
These results suggest that SC was covalently linked to IgA intracellularly just prior to the time of secretion. In the parental cell line chimeric IgA1 dimerizes late in the secretory pathway (11), presumably when J chain is incorporated into the molecule (15). In vivo, sIgA is assembled in the transcytotic pathway of epithelial cells (16–18). The assembly of sIgA in the transfected myeloma cells appears to take place in the Golgi apparatus when dIgA and SC are present together. When culture supernatant from a transfectant was concentrated 100-fold and analyzed by gel filtration on two Superose 6 columns (Pharmacia) in series (19), three overlapping peaks with retention volumes of 27.5, 29.5, and 33 ml were observed (Fig. 2A). When the fractions were analyzed by ELISA all three peaks were found to contain antibody. Anti-SC reacted most strongly with peak I, indicating association of SC with IgA. Supernatants from cells producing only IgA1 yielded two peaks corresponding to dIgA and monomeric IgA (mIgA). No reactivity was seen with anti-SC.
Figure 2.
Analysis of the composition of proteins secreted by transfectants synthesizing chimeric sIgA1 (A) or IgA1 (B). Three hundred microliters of 100-fold concentrated serum-free medium was separated on the basis of molecular weight on two Pharmacia Superose 6 columns in series. The lines with no symbols indicate the elution profiles at 280 nm. Fractions of 0.75 ml were collected and analyzed by ELISA, and IgA was captured on dansylated-BSA-coated microtiter plates that were detected with rabbit anti-κ (squares) (Sigma) or anti-SC (circles), followed by goat anti-rabbit antibody conjugated to alkaline-phosphatase and substrate. The resulting O.D. at 410 nm is plotted. Closed symbols indicate sIgA and open symbols indicate IgA1. The presence of dIgA and mIgA in the peaks was confirmed by analysis of the fractions by SDS/PAGE.
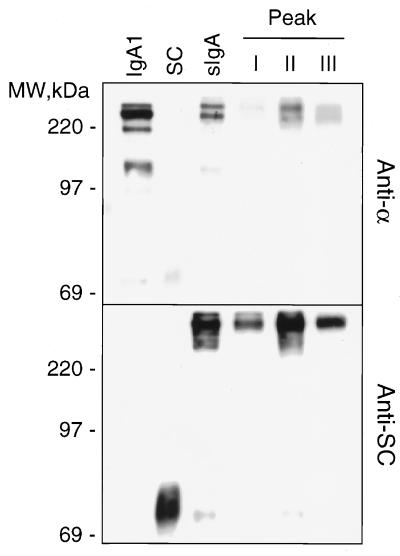
To further characterize the peaks and to determine whether covalent bonds were formed between dIgA and SC in sIgA, the fractions from each of the peaks were concentrated and analyzed by SDS/PAGE and Western blotting (Fig. 3). Anti-α antibodies detected a band with an apparent molecular mass of 400 kDa in peak I and two bands of apparent molecular mass of 400 kDa and 320 kDa in peaks II and III and in the starting material. The 320-kDa band was also seen in supernatants from cells synthesizing only IgA1. Anti-SC antiserum detected the 400-kDa species in all three peaks, indicating that it corresponds to covalently associated sIgA, with the 320-kDa band representing dIgA without attached SC. Free SC is seen in the supernatants of cell lines that produce only SC. It is noteworthy that only a small amount of an 80-kDa protein corresponding to free SC was detected in both the unfractionated sIgA and in peak II, indicating that the majority of SC synthesized by the transfectant is covalently associated with IgA. In vivo, IgA can be found both covalently and noncovalently attached to SC (20, 21).
Figure 3.
Western blot analysis of fast protein liquid chromatography (FPLC) fractions. Fractions I, II, and III from the FPLC analysis shown in Fig. 2 were pooled and concentrated. The proteins were separated by SDS/PAGE in 6% Tris⋅glycine gels and transferred to nitrocellulose. Nonspecific sites were blocked by incubating the blots for 2 hr at room temperature in PBS containing 0.1% Tween-20 and 5% dried milk (PBST-milk). Blots first were incubated for 2 hr at room temperature with rabbit anti-SC in PBST-milk. The specifically bound rabbit antibodies were detected using goat anti-rabbit antibodies conjugated to peroxidase followed by incubation for 1 min with ECL reagent (Amersham) and exposure to Kodak XAR film for 30 sec. After probing with the the first antibody, blots were incubated for 30 min at 50°C in 62.5 mM Tris⋅HCl, pH 6.7, containing 100 mM 2-mercaptoethanol and 2% SDS to remove blot-associated antibodies. Blots were then analyzed with rabbit anti-α chain (Sigma) as described above. Included for comparison are supernatants from transfectants synthesizing only IgA1 or human SC as well as the unfractionated culture supernatant from the cell line synthesizing sIgA.
In Vivo Stability of sIgA.
To determine their in vivo stability, dIgA and sIgA proteins purified from culture supernatants by dansyl-Sepharose affinity chromatography were radiolabeled with 125I and introduced into the stomach of BALB/c mice by intubation. When the elimination of IgA from the mice was followed by whole body counting (22), dIgA was more rapidly eliminated than sIgA (Fig. 4A). At 150 min postintubation, mice were killed and the intestinal contents were isolated and processed. When the protein-bound radioactivity was determined by TCA precipitation, 24.6 × 104 cpm (7.2% of the intubated dIgA) of dIgA and 52.0 × 104 cpm (16.3% of the intubated sIgA) of sIgA were recovered in intestinal washes (Table 1), indicating that more of the intact sIgA could be recovered from the intestinal washes. Consistent with more of the injected sIgA remaining intact in the intestine, a mixture of anti-α and anti-κ chain antisera precipitated about 19.3 × 104 cpm of the sIgA but only 5.0 × 104 cpm of the dIgA (Table 1) from the intestinal washes. Similarly, antigen (DNS-BSA-Sepharose) precipitated 10.4 × 104 cpm of the recovered sIgA but only 2.0 × 104 cpm of the recovered dIgA. SDS/PAGE analysis of the IgA precipitated with antigen showed a major band of 55–60 kDa corresponding to Fab fragments in intestinal washes from mice given either sIgA or dIgA (Fig. 4B, lanes 6 and 7). The immunoprecipitates of anti-α and anti-κ chain showed a major band of 55–65 kDa corresponding to Fab and Fc fragments, with some minor higher molecular mass bands (Fig. 4B, lanes 3 and 4). The slower rate of elimination coupled with the recovery of more total and antigen-specific sIgA than dIgA suggest that sIgA is more stable in the intestines than dIgA. However, both dIgA and sIgA appear to be susceptible to enzymes that cleave in the hinge region.
Table 1.
Recovery of iodinated IgA
| Protein | TCA-precipitable cpm (104)
|
Recovered cpm precipitated (104)
|
||
|---|---|---|---|---|
| Intubated | Recovered (% of intubated) | DNS-BSASepharose | Anti-α + anti-κ | |
| dIgA | 343 | 24.6 (7.2) | 2.0 | 5.0 |
| sIgA | 320 | 52.0 (16.3) | 10.4 | 19.3 |
Two and a half hours after intubation of iodinated dIgA and sIgA into the stomach, mice were killed and the intestinal washes were collected. dIgA and sIgA in intestinal washes were precipitated with 10% TCA and with antibodies, as described in Materials and Methods.
In mice, serum dIgA is transported into bile by the pIgR expressed on the hepatocytes, and this biliary IgA is emptied into the small intestines. To compare the stability of in vivo assembled sIgA with that of sIgA assembled by our transfectant, radiolabeled dIgA1 was injected i.v. into the tail vein of BALB/c mice. Three hours after injection, mice were killed and the intestinal contents isolated. The antigen-specific IgA precipitated from the intestinal washings showed a major band of 55–60 kDa corresponding to Fab (Fig. 4B, lane 5), similar to that found when dIgA or sIgA were introduced directly into the gastrointestinal tract. These results further confirm that the sIgA assembled in a single-cell system is similar to sIgA assembled in vivo.
The development of a single mammalian cell system secreting sIgA makes it possible to produce the quantities of sIgA required for passive immunotherapy and represents a major advance over other methods for producing sIgA (8, 9). This expression system, which uses human α constant region and human SC, also represents a major improvement on the previous attempts to produce sIgA in Nicotiana tabacum plants (23). In plants, rabbit SC, and a hybrid H chain with CH1 and CH2 from murine IgG1 and CH2 and CH3 from murine IgA had been used. Although a multimeric Ig was produced that contained SC, after reduction the SC was smaller than expected. In addition, plant cells attach different complex carbohydrates than do mammalian cells, possibly changing the biological properties of the resulting sIgA. The current use of human κ, α, and SC genes renders the resulting sIgA mostly human and, therefore, potentially more useful for in vivo therapy. Production of sIgA2, which lacks the protease-sensitive hinge region of IgA1, may further enhance the in vivo stability. Additionally, the large number of available IgA-producing hybridomas with various pathogen specificities can be directly transfected with SC, yielding hybridomas that produce sIgA. With slight changes in the expression vectors or expression cell line, totally human sIgA could be produced in single-cell tissue culture systems. Mammalian cells provide a means to produce sIgA in large quantities using established methods.
Acknowledgments
This work was supported by National Institutes of Health Fellowship AI08994 to K.R.C. and by National Institutes of Health Grants CA16858, AI29470, and AI39187 to S.L.M.
ABBREVIATIONS
- sIgA
secretory IgA
- pIgA
polymeric IgA
- mIgA
monomeric IgA
- SC
secretory component
- pIgR
polymeric immunoglobulin receptor
- IMDM
Iscove’s modified Dulbecco’s medium
- DNS-BSA-Sepharose
dansylated BSA coupled to Sepharose beads
References
- 1.Mestecky J, McGhee J R. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- 2.Mostov K E. Annu Rev Immunol. 1994;12:63–84. doi: 10.1146/annurev.iy.12.040194.000431. [DOI] [PubMed] [Google Scholar]
- 3.Mazanec M B, Nedrud J G, Lamm M E. J Virol. 1987;61:2624–2626. doi: 10.1128/jvi.61.8.2624-2626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazanec M B, Nedrud J G, Liang X P, Lamm M E. J Immunol. 1989;142:4275–4281. [PubMed] [Google Scholar]
- 5.Renegar K B, Small P A. J Immunol. 1991;146:1972–1978. [PubMed] [Google Scholar]
- 6.Brown W R, Newcomb R W, Ishizaka K. J Clin Invest. 1970;49:1374–1380. doi: 10.1172/JCI106354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindh E. J Immunol. 1975;114:284–286. [PubMed] [Google Scholar]
- 8.Hirt R P, Hughes G J, Frutiger S, Michetti P, Perregaux C, Poulain-Godefroy O, Jeanguenat N, Neutra M R, Kraehenbuhl J P. Cell. 1993;74:245–255. doi: 10.1016/0092-8674(93)90416-n. [DOI] [PubMed] [Google Scholar]
- 9.Lullau, E., Heyse, S., Vogel, H., Marison, I., von Stockar, U., Kraehenbuhl, J. P. & Corthesy, B. J. Biol. Chem., 271, 16300–16309. [DOI] [PubMed]
- 10.Tamer C M, Lamm M E, Robinson J K, Piskurich J F, Kaetzel C S. J Immunol. 1995;155:707–714. [PubMed] [Google Scholar]
- 11.Chintalacharuvu K R, Morrison S L. J Immunol. 1996;157:3443–3449. [PubMed] [Google Scholar]
- 12.Chintalacharuvu K R, Piskurich J, Lamm M E, Kaetzel C S. J Cell Physiol. 1991;148:35–47. doi: 10.1002/jcp.1041480105. [DOI] [PubMed] [Google Scholar]
- 13.Mostov K E, Blobel G. Methods Enzymol. 1983;98:458–466. doi: 10.1016/0076-6879(83)98173-9. [DOI] [PubMed] [Google Scholar]
- 14.Elson C O, Ealding W, Lefkovitz J. J Immunol Methods. 1984;67:101–108. doi: 10.1016/0022-1759(84)90089-9. [DOI] [PubMed] [Google Scholar]
- 15.Koshland M E. Annu Rev Immunol. 1985;3:425–453. doi: 10.1146/annurev.iy.03.040185.002233. [DOI] [PubMed] [Google Scholar]
- 16.Brandtzaeg P. Scan J Immunol. 1978;8:39–52. doi: 10.1111/j.1365-3083.1978.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 17.Brandtzaeg P, Prydz H. Nature (London) 1984;311:71–73. doi: 10.1038/311071a0. [DOI] [PubMed] [Google Scholar]
- 18.Chintalacharuvu K R, Tavill A S, Louis L N, Vaerman J-P, Lamm M E, Kaetzel C S. Hepatology. 1993;19:162–173. [PubMed] [Google Scholar]
- 19.Chintalacharuvu K R, Lamm M E, Kaetzel C S. Mol Immunol. 1994;30:19–26. doi: 10.1016/0161-5890(93)90422-8. [DOI] [PubMed] [Google Scholar]
- 20.Schneiderman R D, Hanley W C, Knight K L. Proc Natl Acad Sci USA. 1989;86:7561–7565. doi: 10.1073/pnas.86.19.7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knight K L, Malek T R, Dray S. J Immunol. 1975;115:595–598. [PubMed] [Google Scholar]
- 22.Zuckier L S, Georgescu L, Chang C J, Scharff M D, Morrison S L. Cancer. 1994;73:794–797. doi: 10.1002/1097-0142(19940201)73:3+<794::aid-cncr2820731308>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 23.Ma J K-C, Hiatt A, Hein M, Vine N B, Wang F, Stabila P, van Bolleweerd C, Mostov K E, Lehner T. Science. 1995;268:716–719. doi: 10.1126/science.7732380. [DOI] [PubMed] [Google Scholar]