Abstract
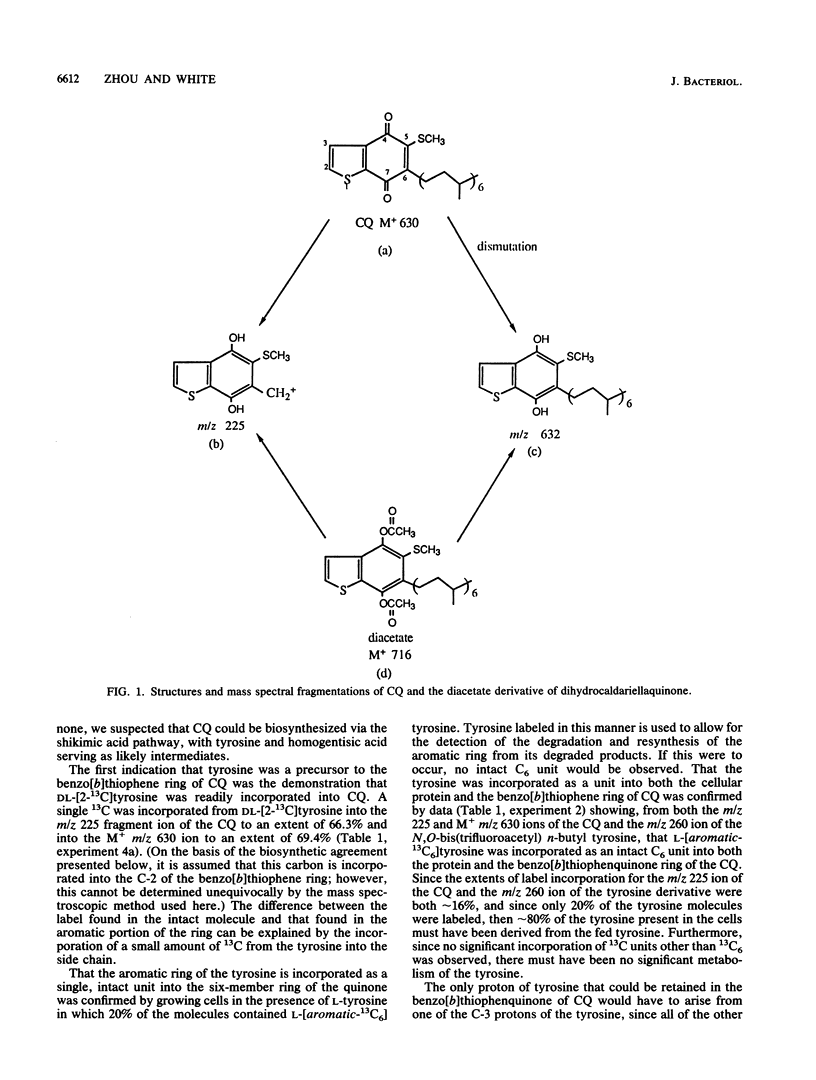
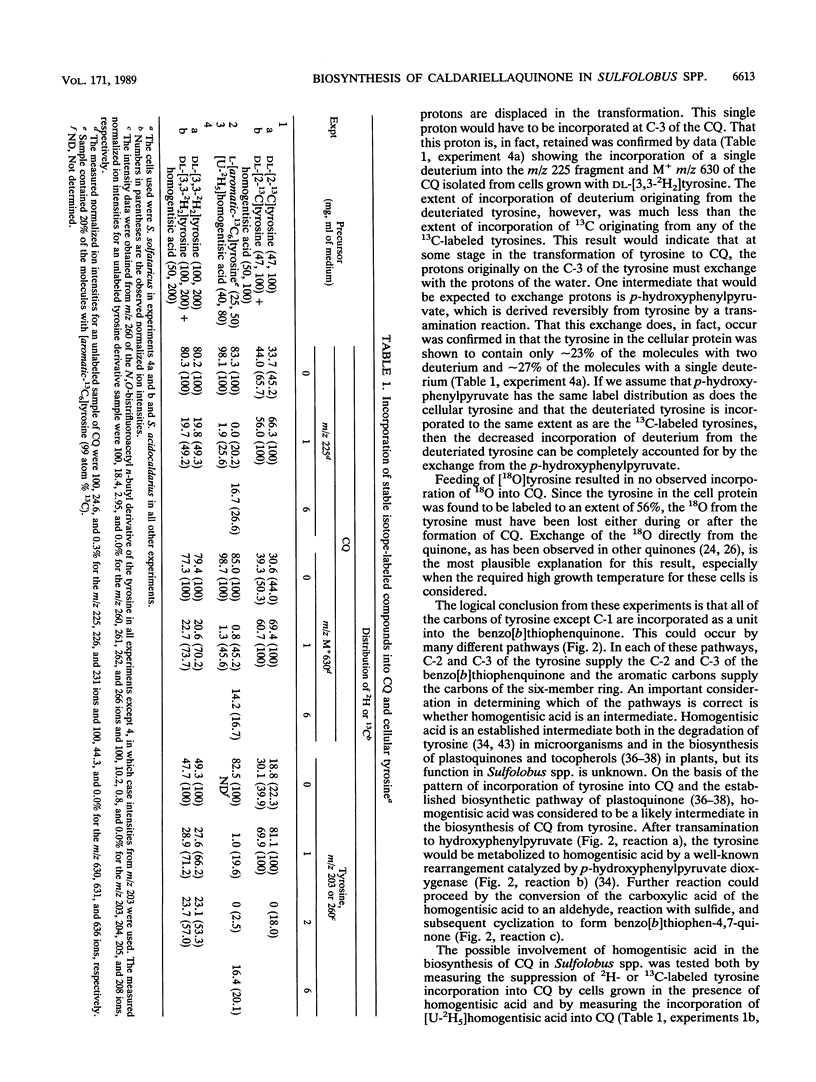
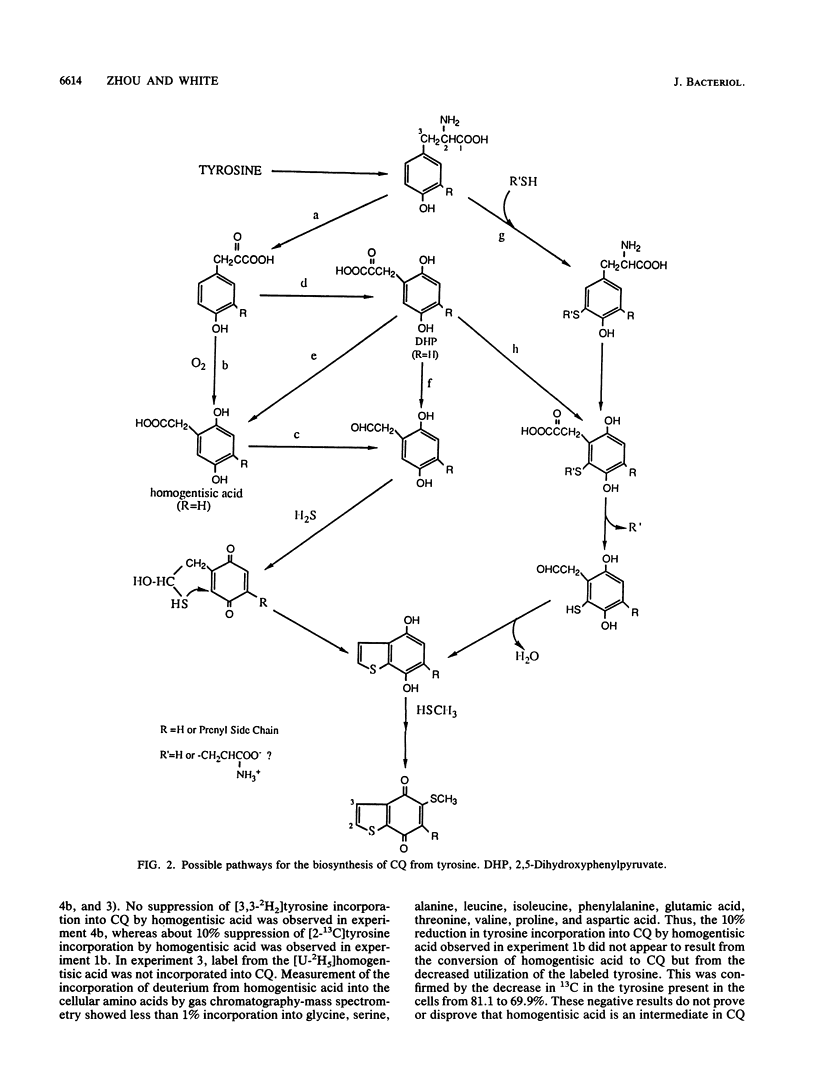
The biosynthesis of caldariellaquionone (CQ) was studied in species of Sulfolobus by measuring the incorporation of stable isotopically labeled tyrosines into CQ. By feeding a series of tyrosines labeled with deuterium or 13C and then measuring the extent and position at which label was incorporated into CQ by mass spectrometry, it was shown that more than 95% of the label was incorporated into the benzo[b]thiophen-4,7-quinone moiety of CQ. From the labeling experiments, it is concluded that the benzo[b]thiophen-4,7-quinone is derived as an intact unit from all of the carbons of tyrosine except C-1.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley R., Meganathan R. Biosynthesis of vitamin K (menaquinone) in bacteria. Microbiol Rev. 1982 Sep;46(3):241–280. doi: 10.1128/mr.46.3.241-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D., Brock K. M., Belly R. T., Weiss R. L. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Mikrobiol. 1972;84(1):54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- Campbell I. M., Robins D. J., Kelsey M., Bentley R. Biosynthesis of bacterial menaquinones (vitamins K 2 ). Biochemistry. 1971 Aug 3;10(16):3069–3078. doi: 10.1021/bi00792a014. [DOI] [PubMed] [Google Scholar]
- Collins M. D., Jones D. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implication. Microbiol Rev. 1981 Jun;45(2):316–354. doi: 10.1128/mr.45.2.316-354.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B. C., Lounasmaa M., Tendille C., Lederer E. Mass spectrometry of plastoquinones. The structure of the plastoquinones B,C and D. Biochem Biophys Res Commun. 1965 Nov 22;21(4):318–322. doi: 10.1016/0006-291x(65)90195-6. [DOI] [PubMed] [Google Scholar]
- De Rosa M., De Rosa S., Gambacorta A., Minale L. Caldariellaquinone, a unique benzo(b)thiophen-4,7-quinone from Caldariella acidophila, an extremely thermophilic and acidophilic bacterium. J Chem Soc Perkin 1. 1977;(6):653–657. doi: 10.1039/p19770000653. [DOI] [PubMed] [Google Scholar]
- Eckert H., Fiat D. Isotopic labeling of tyrosine, followed by 17O n.m.r. Int J Pept Protein Res. 1986 Jun;27(6):613–616. doi: 10.1111/j.1399-3011.1986.tb01057.x. [DOI] [PubMed] [Google Scholar]
- GIBSON M. I., GIBSON F. A new intermediate in aromatic biosynthesis. Biochim Biophys Acta. 1962 Nov 19;65:160–163. doi: 10.1016/0006-3002(62)90166-x. [DOI] [PubMed] [Google Scholar]
- Gibson F., Pittard J. Pathways of biosynthesis of aromatic amino acids and vitamins and their control in microorganisms. Bacteriol Rev. 1968 Dec;32(4 Pt 2):465–492. [PMC free article] [PubMed] [Google Scholar]
- Hack M. H., Helmy F. M. The melanins and lipofuscin. Comp Biochem Physiol B. 1983;76(3):399–407. doi: 10.1016/0305-0491(83)90266-3. [DOI] [PubMed] [Google Scholar]
- Jefford C. W., Cadby P. A. Evaluation of models for the mechanism of action of 4-hydroxyphenylpyruvate dioxygenase. Experientia. 1981 Nov 15;37(11):1134–1137. doi: 10.1007/BF01989880. [DOI] [PubMed] [Google Scholar]
- Lanzotti V., Trincone A., Gambacorta A., De Rosa M., Breitmaier E. 1H and 13C NMR assignment of benzothiophenquinones from the sulfur-oxidizing archaebacterium Sulfolobus solfataricus. Eur J Biochem. 1986 Oct 1;160(1):37–40. doi: 10.1111/j.1432-1033.1986.tb09936.x. [DOI] [PubMed] [Google Scholar]
- Lawrence J., Cox G. B., Gibson F. Biosynthesis of ubiquinone in Escherichia coli K-12: biochemical and genetic characterization of a mutant unable to convert chorismate into 4-hydroxybenzoate. J Bacteriol. 1974 Apr;118(1):41–45. doi: 10.1128/jb.118.1.41-45.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinberger R., Hull W. E., Simon H., Rétey J. Steric course of the NIH shift in the enzymic formation of homogentisic acid. Eur J Biochem. 1981 Jul;117(2):311–318. doi: 10.1111/j.1432-1033.1981.tb06338.x. [DOI] [PubMed] [Google Scholar]
- Lindblad B., Lindstedt G., Lindstedt S. The mechanism of enzymic formation of homogentisate from p-hydroxyphenylpyruvate. J Am Chem Soc. 1970 Dec 16;92(25):7446–7449. doi: 10.1021/ja00728a032. [DOI] [PubMed] [Google Scholar]
- Olson R. E., Rudney H. Biosynthesis of ubiquinone. Vitam Horm. 1983;40:1–43. doi: 10.1016/s0083-6729(08)60431-8. [DOI] [PubMed] [Google Scholar]
- Snyder C. D., Rapoport H. Biosynthesis of bacterial menaquinones. Origin of quinone oxygens. Biochemistry. 1970 May 12;9(10):2033–2038. doi: 10.1021/bi00812a001. [DOI] [PubMed] [Google Scholar]
- Thomson R. H. The pigments of reddish hair and feathers. Angew Chem Int Ed Engl. 1974 May;13(5):305–312. doi: 10.1002/anie.197403051. [DOI] [PubMed] [Google Scholar]
- Thorne K. J. Incorporation of radioactive mevalonate into C50 and C55 phenols by Streptococcus mutans. Biochem J. 1973 Nov;135(3):567–568. doi: 10.1042/bj1350567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne K. J., Kodicek E. The structure of bactoprenol, a lipid formed by lactobacilli from mevalonic acid. Biochem J. 1966 Apr;99(1):123–127. doi: 10.1042/bj0990123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurl S., Witke W., Buhrow I., Schäfer W. Quinones from archaebacteria, II. Different types of quinones from sulphur-dependent archaebacteria. Biol Chem Hoppe Seyler. 1986 Mar;367(3):191–197. doi: 10.1515/bchm3.1986.367.1.191. [DOI] [PubMed] [Google Scholar]
- Whistance G. R., Threlfall D. R. Biosynthesis of phytoquinones. Biosynthetic origins of the nuclei and satellite methyl groups of plastoquinone, tocopherols and tocopherolquinones in maize shoots, bean shoots and ivy leaves. Biochem J. 1968 Oct;109(4):577–595. doi: 10.1042/bj1090577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistance G. R., Threlfall D. R. Biosynthesis of phytoquinones. Homogentisic acid: a precursor of plastoquinones, tocopherols and alpha-tocopherolquinone in higher plants, green algae and blue-green algae. Biochem J. 1970 Apr;117(3):593–600. doi: 10.1042/bj1170593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistance G. R., Threlfall D. R. Biosynthesis of phytoquinones: utilization of homogentisic acid by maize shoots for the biosynthesis of plastoquinone. Biochem J. 1968 Sep;109(3):482–483. doi: 10.1042/bj1090482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. H. 4-Hydroxybenzyl alcohol. A metabolite produced during the biosynthesis of thiamine in Escherichia coli. Biochim Biophys Acta. 1979 Feb 19;583(1):55–62. doi: 10.1016/0304-4165(79)90309-x. [DOI] [PubMed] [Google Scholar]



