Abstract
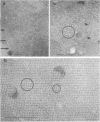
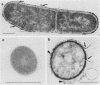
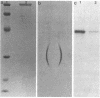
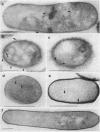
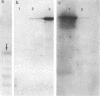
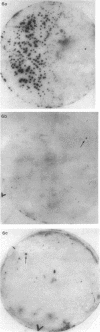
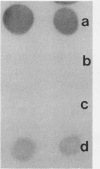
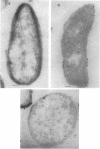
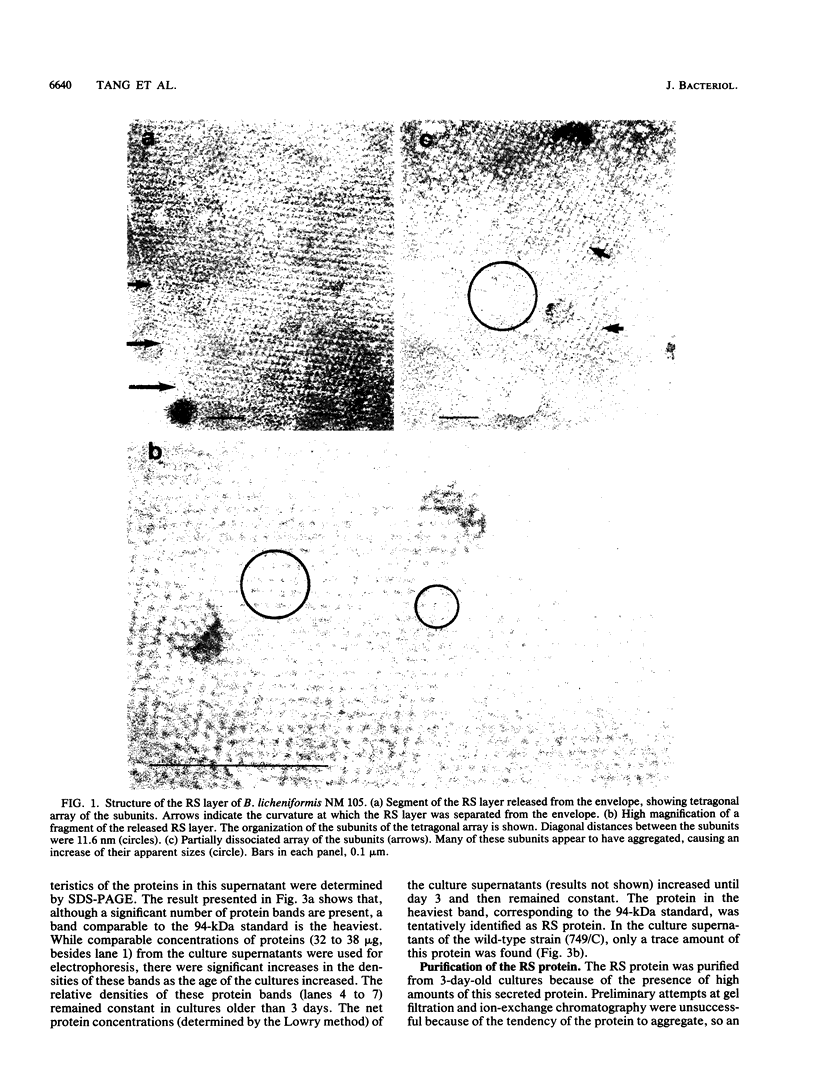
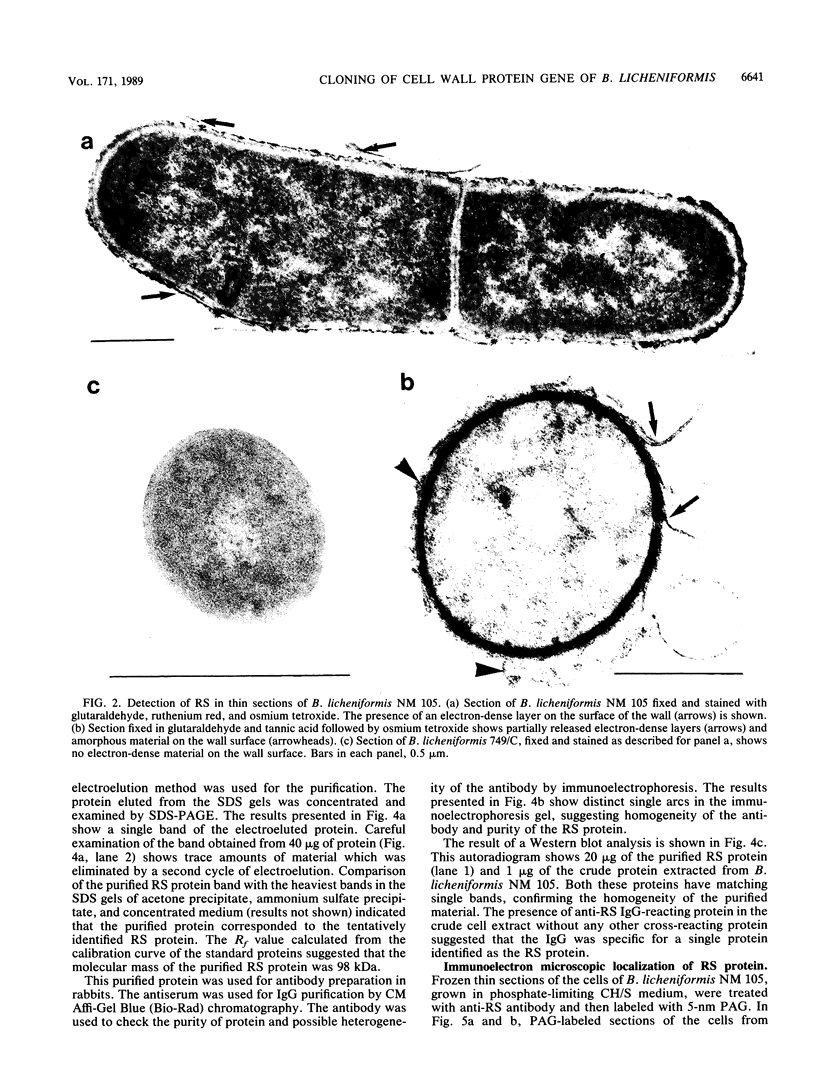
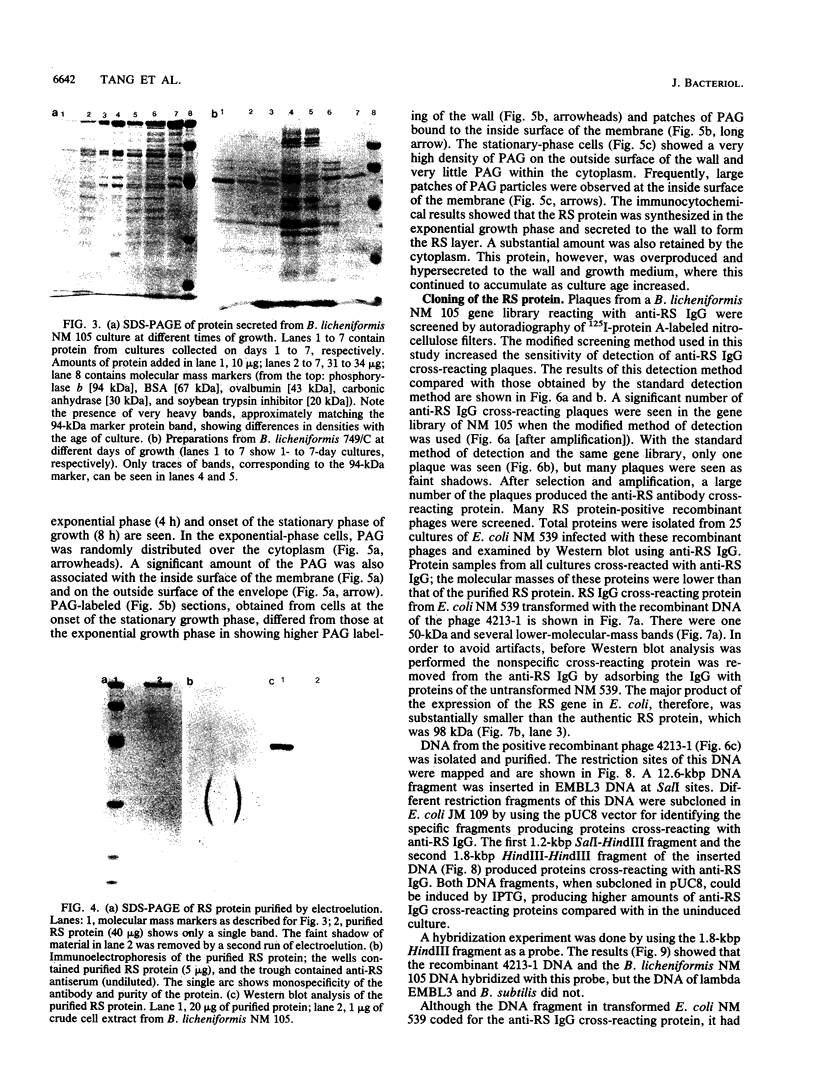
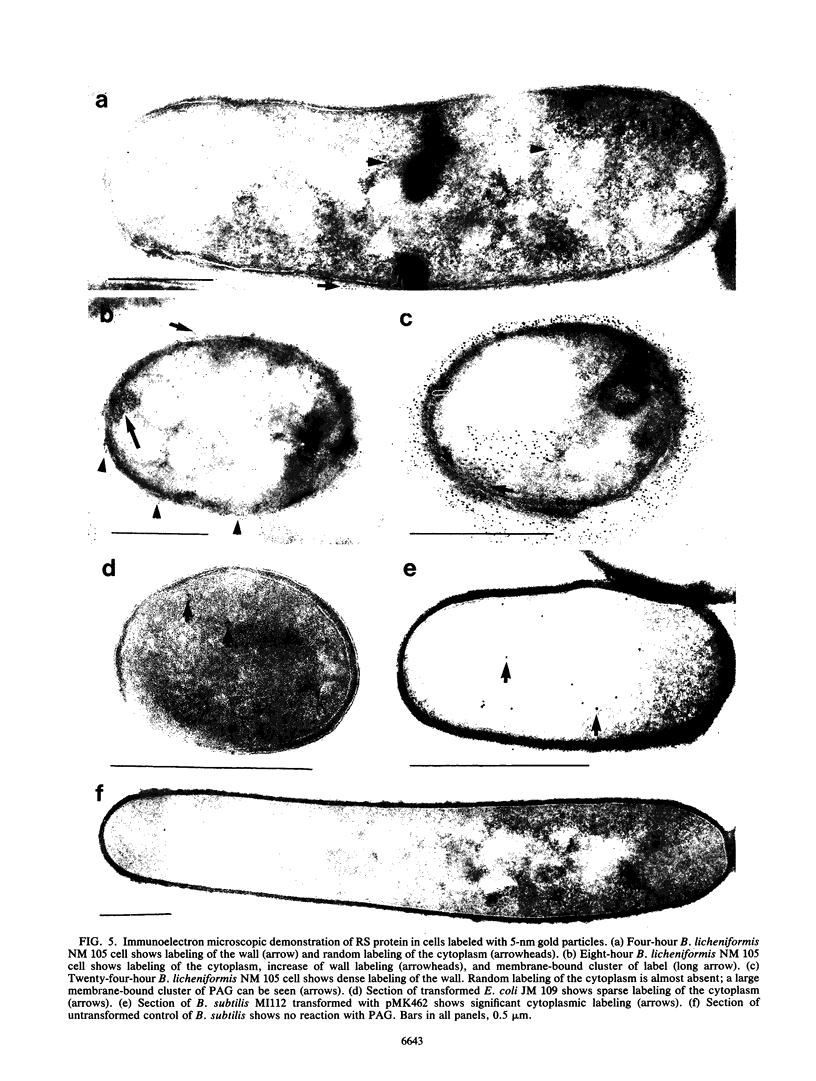
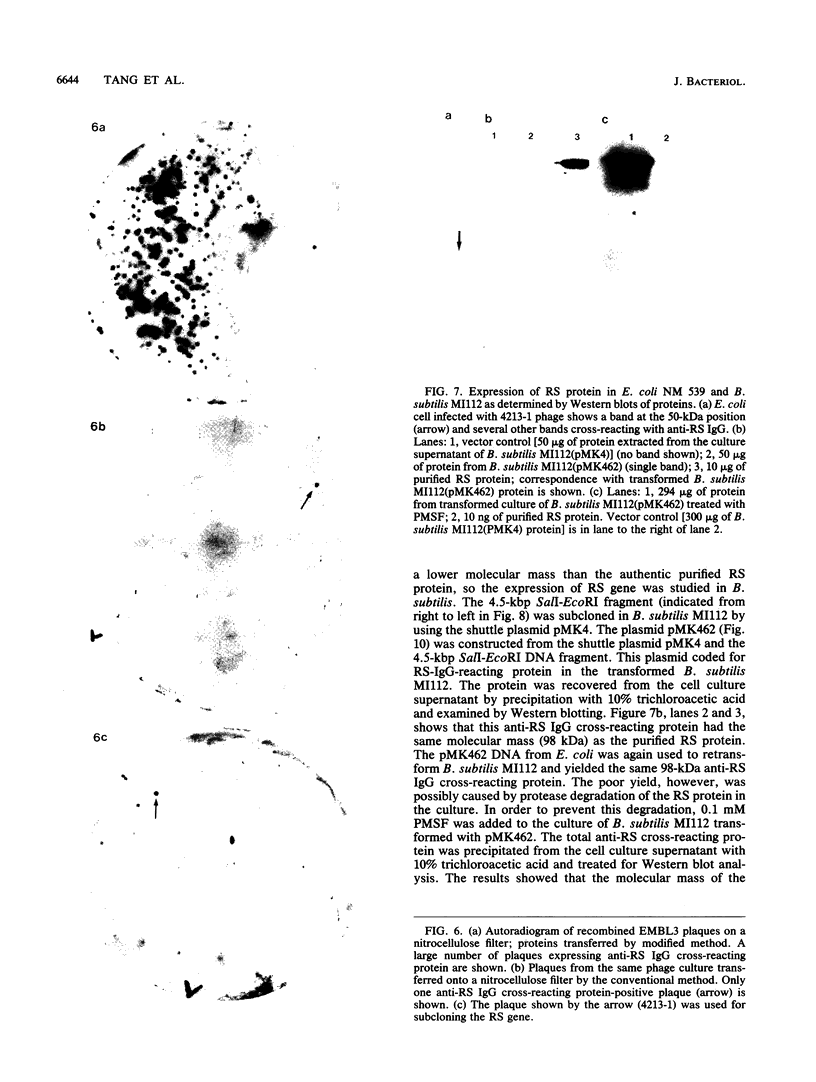
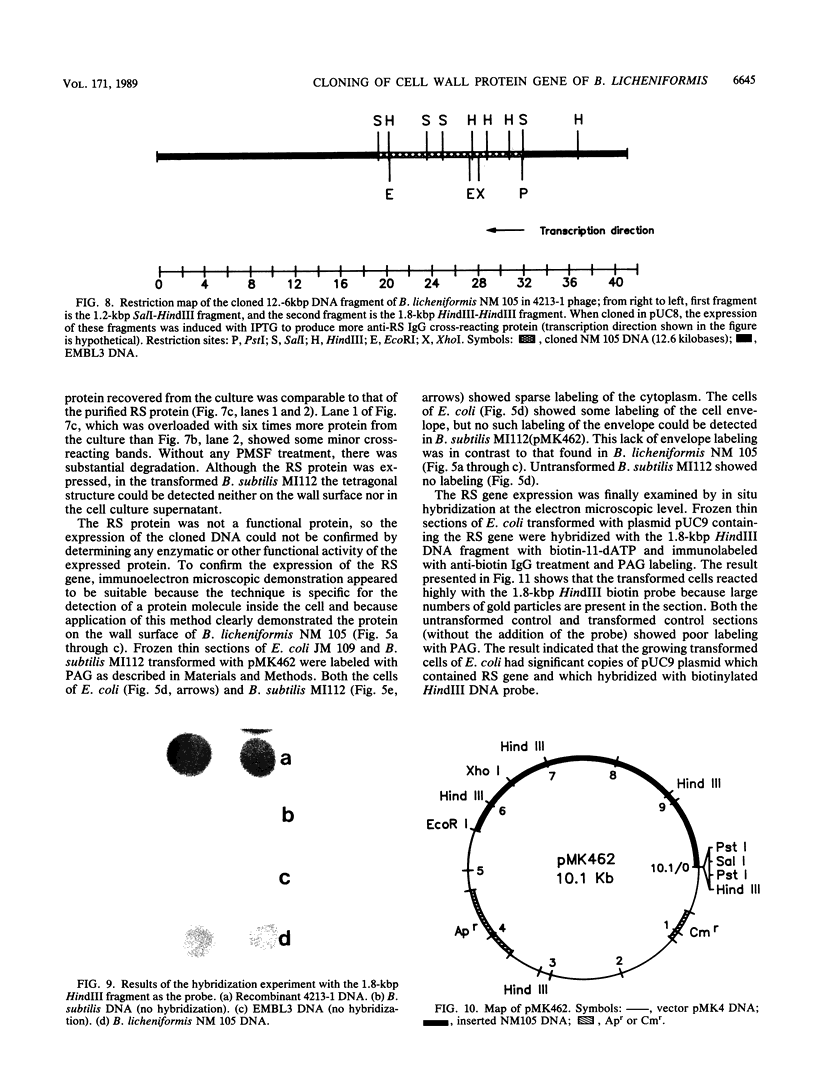
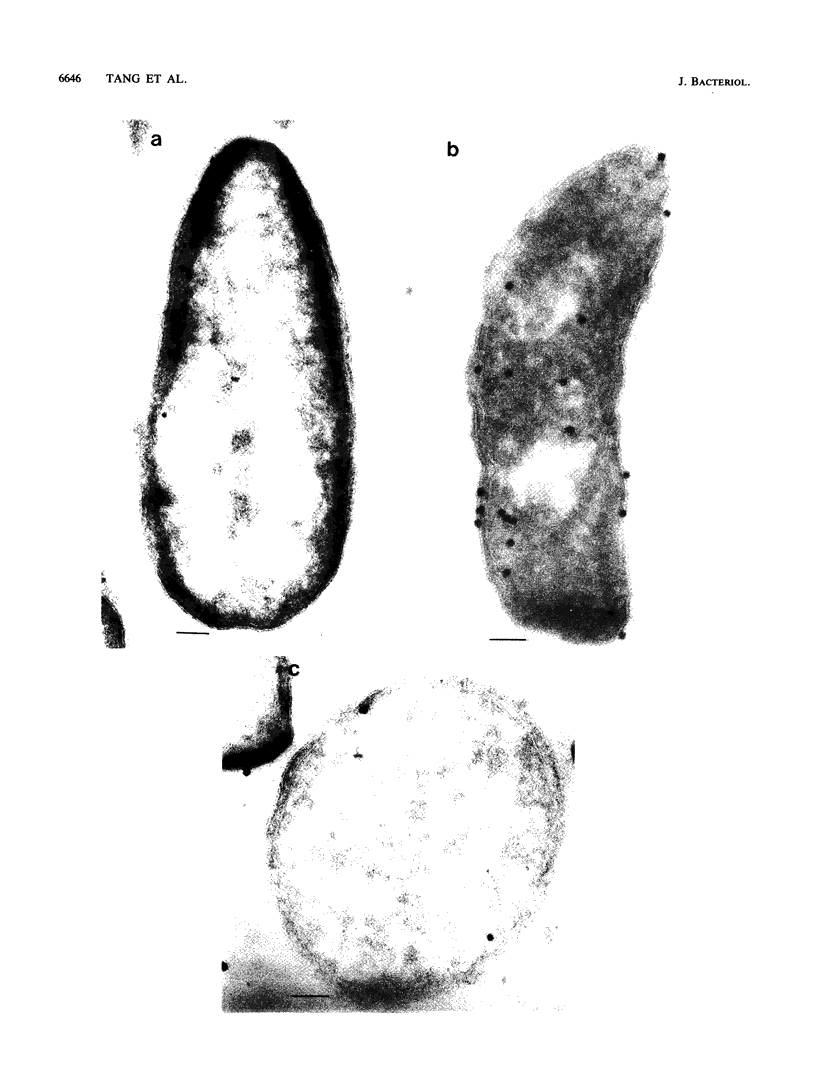
A protein with a tetragonal pattern, defined as RS protein, was found on the wall surface of an alkaline phosphatase secretion-deficient mutant (NM 105) of Bacillus licheniformis 749/C. The protein was present on the wall surface of the exponential-growth-phase cells, but at the stationary growth phase it was overproduced and hypersecreted. This protein was precipitated to homogeneity from the culture fluid by 80% ammonium sulfate saturation and chilled acetone. The molecular mass of the protein was 98 kilodaltons, and it had a single subunit in a sodium dodecyl sulfate gel. Specific anti-RS antibody was generated in rabbits and used to immunolabel the RS protein on the cells at different growth phases. In early-exponential-growth-phase cells, the outside surface of the wall, the cytoplasm, and the inside surface of the cytoplasmic membrane were labeled. In stationary-growth-phase cells, the cytoplasm was poorly labeled, but the labeling on the outside surface of the wall was high. AB. licheniformis NM 105 gene library was made by using the lambda phage EMBL3. The RS protein expression from this gene library was detected by a modified autoradiographic procedure. One of the amplified RS protein-positive plaques (4213-1) containing recombinant DNA was chosen, and the restriction map of this DNA was prepared. The RS protein expressed in Escherichia coli NM 539 infected with 4213-1 recombinant phage had a lower molecular mass than the purified authentic RS protein. The 4.5-kilobase-pair (kbp) SalI-EcoRI fragment of the recombinant DNA was cloned in the shuttle plasmid pMK4 to construct pMK462, which was expressed in B. subtilis MI112 and produced the RS protein identical in molecular mass to the purified authentic RS protein. The RS protein expression was also demonstrated in cryosections of transformed E. coli and B. subtilis cells by immunoelectron microscopy. The 1.2-kbp SalI-HindIII and 1.8-kbp HindIII-HindIII recombinant DNA restriction enzyme fragments, respectively, from the right of the restriction map produced anti-RS antibody cross-reacting proteins. The expression of the 1.2-kbp SalI-HindIII DNA fragment cloned in pUC8 could be induced with isopropyl-beta-D-thiogalactopyranoside. The 1.8-kbp DNA restriction fragment hybridized with both the chromosomal DNA of strain NM 105 and the recombinant phage 4213-1 DNA. The RS gene expression was finally demonstrated in transformed E. coli 539 cells by in situ hybridization of frozen thin sections with the 1.8-kbp HindIII biotin-dATP probe and immunolabeling these with anti-biotin immunoglobulin G and protein A-gold.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beveridge T. J. Surface arrays on the wall of Sporosarcina ureae. J Bacteriol. 1979 Sep;139(3):1039–1048. doi: 10.1128/jb.139.3.1039-1048.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder M., Tourmente S., Roth J., Renaud M., Gehring W. J. In situ hybridization at the electron microscope level: localization of transcripts on ultrathin sections of Lowicryl K4M-embedded tissue using biotinylated probes and protein A-gold complexes. J Cell Biol. 1986 May;102(5):1646–1653. doi: 10.1083/jcb.102.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Evenberg D., Van Boxtel R., Lugtenberg B., Schurer F., Blommaert J., Bootsma R. Cell surface of the fish pathogenic bacterium Aeromonas salmonicida. I. Relationship between autoagglutination and the presence of a major cell envelope protein. Biochim Biophys Acta. 1982 Jan 22;684(2):241–248. doi: 10.1016/0005-2736(82)90012-8. [DOI] [PubMed] [Google Scholar]
- Evenberg D., Van Boxtel R., Lugtenberg B., Schurer F., Blommaert J., Bootsma R. Cell surface of the fish pathogenic bacterium Aeromonas salmonicida. I. Relationship between autoagglutination and the presence of a major cell envelope protein. Biochim Biophys Acta. 1982 Jan 22;684(2):241–248. doi: 10.1016/0005-2736(82)90012-8. [DOI] [PubMed] [Google Scholar]
- Ghosh B. K., Owens K., Pietri R., Peterkofsky A. Localization to the inner surface of the cytoplasmic membrane by immunoelectron microscopy of enzyme I of the phosphoenolpyruvate:sugar phosphotransferase system of Escherichia coli. Proc Natl Acad Sci U S A. 1989 Feb;86(3):849–853. doi: 10.1073/pnas.86.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan T., Ghosh A., Ghosh B. K. Immunoelectron microscopic double labeling of alkaline phosphatase and penicillinase with colloidal gold in frozen thin sections of Bacillus licheniformis 749/C. J Bacteriol. 1985 Oct;164(1):107–113. doi: 10.1128/jb.164.1.107-113.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOUWINK A. L. A macromolecular mono-layer in the cell wall of Spirillum spec. Biochim Biophys Acta. 1953 Mar;10(3):360–366. doi: 10.1016/0006-3002(53)90266-2. [DOI] [PubMed] [Google Scholar]
- Hydrean C., Ghosh A., Nallin M., Ghosh B. K. Interrelationship of carbohydrate metabolism and alkaline phosphatase synthesis in Bacillus licheniformis 749/c. J Biol Chem. 1977 Oct 10;252(19):6806–6812. [PubMed] [Google Scholar]
- Kumar R., Ghosh A., Ghosh B. K. Alkaline phosphatase secretion-negative mutant of Bacillus licheniformis 749/C. J Bacteriol. 1983 May;154(2):946–954. doi: 10.1128/jb.154.2.946-954.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nermut M. V., Murray R. G. Ultrastructure of the cell wall of Bacillus polymyxa. J Bacteriol. 1967 Jun;93(6):1949–1965. doi: 10.1128/jb.93.6.1949-1965.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaudet B., Ehrlich S. D. In vitro genetic labeling of Bacillus subtilis cryptic plasmid pHV400. Plasmid. 1979 Jan;2(1):48–58. doi: 10.1016/0147-619x(79)90005-2. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B., Glauert A. M. Ultrastructure of the cell walls of two closely related clostridia that possess different regular arrays of surface subunits. J Bacteriol. 1976 May;126(2):869–882. doi: 10.1128/jb.126.2.869-882.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers on bacteria. Annu Rev Microbiol. 1983;37:311–339. doi: 10.1146/annurev.mi.37.100183.001523. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B. Regular arrays of macromolecules on bacterial cell walls: structure, chemistry, assembly, and function. Int Rev Cytol. 1978;53:1–62. doi: 10.1016/s0074-7696(08)62240-8. [DOI] [PubMed] [Google Scholar]
- Stewart M., Beveridge T. J., Murray R. G. Structure of the regular surface layer of Spirillum putridiconchylium. J Mol Biol. 1980 Feb 15;137(1):1–8. doi: 10.1016/0022-2836(80)90153-9. [DOI] [PubMed] [Google Scholar]
- Stewart M., Beveridge T. J. Structure of the regular surface layer of Sporosarcina ureae. J Bacteriol. 1980 Apr;142(1):302–309. doi: 10.1128/jb.142.1.302-309.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornley M. J., Glauert A. M., Sleytr U. B. Isolation of outer membranes with an ordered array of surface subunits from Acinetobacter. J Bacteriol. 1973 Jun;114(3):1294–1308. doi: 10.1128/jb.114.3.1294-1308.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinglu G., Ghosh A., Ghosh B. K. Subcellular localization of alkaline phosphatase in Bacillus licheniformis 749/C by immunoelectron microscopy with colloidal gold. J Bacteriol. 1984 Aug;159(2):668–677. doi: 10.1128/jb.159.2.668-677.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi A., Tsukagoshi N., Udaka S. Reassembly in vitro of hexagonal surface arrays in a protein-producing bacterium, Bacillus brevis 47. J Bacteriol. 1982 Sep;151(3):1485–1497. doi: 10.1128/jb.151.3.1485-1497.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi A., Uchihi R., Tabata R., Takahashi Y., Hashiba H., Sasaki T., Yamagata H., Tsukagoshi N., Udaka S. Characterization of the genes coding for two major cell wall proteins from protein-producing Bacillus brevis 47: complete nucleotide sequence of the outer wall protein gene. J Bacteriol. 1986 Oct;168(1):365–373. doi: 10.1128/jb.168.1.365-373.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukagoshi N., Tabata R., Takemura T., Yamagata H., Udaka S. Molecular cloning of a major cell wall protein gene from protein-producing Bacillus brevis 47 and its expression in Escherichia coli and Bacillus subtilis. J Bacteriol. 1984 Jun;158(3):1054–1060. doi: 10.1128/jb.158.3.1054-1060.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland F., Dompert W., Bernhardt G., Sumper M. Halobacterial glycoprotein saccharides contain covalently linked sulphate. FEBS Lett. 1980 Oct 20;120(1):110–114. doi: 10.1016/0014-5793(80)81058-1. [DOI] [PubMed] [Google Scholar]
- Winter A. J., McCoy E. C., Fullmer C. S., Burda K., Bier P. J. Microcapsule of Campylobacter fetus: chemical and physical characterization. Infect Immun. 1978 Dec;22(3):963–971. doi: 10.1128/iai.22.3.963-971.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]