Abstract
Human prostate-specific antigen (PSA) has been widely used as a serum marker for cancer of the prostate. The cell type-specific expression of PSA also makes it a potential tumor antigen for prostate cancer immunotherapy. Study of the immunological aspects of PSA within either normal or malignant prostate tissue has been hampered by the lack of a mouse model, because no PSA counterpart has been identified in mice. Using a 14-kb genomic DNA region that encompasses the entire human PSA gene and adjacent flanking sequences, we generated a series of human PSA transgenic mice. In the six independent lines of transgenic mice generated, the expression of the human PSA transgene, driven by its own cis-acting regulatory elements, is specifically targeted to the prostate. Tissue distribution analysis demonstrated that PSA transgene expression closely follows the human expression pattern. Immunohistochemical analysis of the prostate tissue also showed that the expression of the PSA transgene is confined to the ductal epithelial cells. Despite expressing PSA as a self-antigen in the prostate, these transgenic mice were able to mount a cytotoxic immune response against PSA expressed by tumor cells, indicating that expression of the transgene has not resulted in complete nonresponsiveness. This transgenic mouse model will provide a well defined system to gain an insight into the mechanisms of nonresponsiveness to PSA, ultimately leading to strategies for immunotherapy of human prostate cancer using PSA as the target antigen.
Keywords: prostate-specific antigen, prostate adenocarcinoma, T cell-mediated immunity, tolerance, transgenics
Prostate cancer is the second leading cause of cancer deaths in American men (1). Although this disease is rarely seen in men under the age of 50, the incidence of prostate cancer increases rapidly in subsequent decades of life. Surgery, radiation, and hormonal therapies are the standard treatments for prostate cancer; however, these conventional therapies ultimately are ineffective for metastatic disease (2). Clearly, new treatment strategies are needed to combat the aggressive form of this disease. Immunotherapy mediated through cytotoxic T lymphocytes (CTL) offers a promising treatment avenue, because T cells, in principle, can migrate throughout the body and specifically recognize and destroy metastatic tumor cells in an antigen-specific manner. CTL recognize a complex of self-major histocompatibility complex and endogenously synthesized peptide on the surface of cells. Thus, any endogenously synthesized protein, whether cytoplasmic, membrane-bound, or secreted, can serve as the source of antigenic peptide, which is then displayed on the cell surface. Prostate cancer cells express a well characterized antigen, prostate-specific antigen (PSA), whose expression is widely used clinically as a marker for prostate cancer (3). PSA, a kallikrein with serine protease activity (4), has a highly restricted tissue distribution and is expressed in the normal epithelial cells of the prostate gland, the same cell type from which most prostate tumors arise (5). Neither the regulation of PSA expression nor the role of PSA in normal or neoplastic prostate cells is well understood (5). However, the highly specific expression of PSA makes it a potential target antigen for anti-tumor CTL.
An obvious concern in using PSA as a target antigen for immunotherapy is that it is a self-antigen. To date, much of the work on tumor immunotherapy has implicitly assumed that it is necessary to identify and characterize antigens that are specifically and uniquely expressed in tumors but not in normal tissues. However, this assumption may not be warranted. For example, recent work has revealed that many targets for anti-melanoma CTL, such as tyrosinase, MART-1, gp100, and gp75, are normal self-antigens specific to the melanocyte lineage (6–9). Further, the existence of a number of tissue-specific autoimmune diseases supports the concept that self-reactive immune effectors can be activated under appropriate conditions. Taken together, these findings raise the possibility that tissue-specific differentiation antigens could serve as targets for immunotherapy for other cancers besides melanoma. Thus, it may not be necessary to first isolate anti-tumor CTL from patients to identify the target antigens for characterization, although this approach has been used in melanoma studies (10). Instead, it may be possible to induce a cell-mediated immune response against a normal tissue-specific antigen whether or not such responses typically occur in patients.
The study of PSA as a potential target antigen for immunotherapy, as well as other studies investigating its physiologic role, has been hampered by the lack of appropriate animal models. There is no currently available mouse system to model the salient immunological aspects of prostate cancer, as no prostate-specific kallikrein has been reported for mice. Prior work using transfection assays in cell lines, as well as sequence analysis, had identified several regulatory or transcription elements in the PSA promoter, including an androgen-responsive element (11, 12). However, a limitation of all in vitro approaches is that they cannot determine whether all the necessary elements are present to confer tissue-specific expression in vivo. Therefore, we set out to create a transgenic mouse system that expresses human PSA using a strategy that did not require the previous identification of the tissue-specific regulatory elements. We speculated that a genomic construct of the entire human PSA gene, if it contained the tissue-specific promoter/enhancer elements, would result in prostate-specific expression in mice due to the evolutionary conservation of transcription factors. Using this strategy, we describe in this report the initial characterization of transgenic mice that express human PSA specifically in the prostate. Further, we show this expression does not eliminate the ability of these animals to mount an anti-PSA cytotoxic response to tumors expressing PSA. These findings have significant implications for the regulation of prostate-specific genes and immunological tolerance, as well as for the immunotherapy of prostate cancer.
MATERIALS AND METHODS
Generation of PSA-1 Construct and Transgenic Mice.
A more detailed description of the isolation and characterization of the PSA genomic clones, as well as the construction of the PSA expression vector will be presented elsewhere (unpublished work). In brief, a PSA cDNA clone described previously (13) was used to screen a human lymph node genomic library (ATCC 57760). A lambda clone encompassing the PSA gene was isolated (clone 60–2.1P1). The 5′ end of this clone was then used as a probe to screen a human chromosome 19-specific genomic library (ATCC 57711) to isolate clone 11–1P1. The 6-kb HindIII fragment from the lambda clone 11–1P1 was subcloned into the pBluescript vector (Stratagene) at the HindIII site, and the resulting plasmid was partially digested with HindIII, filled in, and re-ligated to destroy the 5′ HindIII site. Similarly, the 8-kb HindIII fragment from the lambda clone 60–2.1P1 was subcloned into pBluescript in such a way that the 3′ HindIII site was destroyed. These two fragments were then joined together through the preserved HindIII site, resulting in a plasmid clone called pBS/PSA-1. After removing the vector sequence, the PSA-1 transgene construct was microinjected into fertilized embryos from the intercross of (C57BL/6J × DBA/2J) F1 hybrid mice as described (14). Transgenic founders, collectively called PSA1 transgenics, were backcrossed to BALB/cByJ mice (H-2d) to established transgenic lines semi-syngeneic to the PSA-expressing line 1 cells (H-2d) used in the tumor-infiltrating lymphocyte (TIL) experiments described below.
Cell Lines and Transfectants.
Line 1 is a small cell lung carcinoma cell line derived from a female BALB/c mouse (15). Line 1/PSA, a PSA-expressing line 1 transfectant, was generated and characterized previously (13). LNCaP, a human prostatic cell line, was obtained from the American Type Culture Collection (CRL 1740).
DNA and RNA Analyses.
Southern blot analysis to identify mice that have incorporated the transgene and Northern blot analysis to analyze the tissue distribution of the transgene expression were performed as described (16). In both cases, the probe used was the full-length PSA cDNA labeled by the random hexamer method. For reverse transcriptase–PCR (RT-PCR) analysis, total RNA isolated from various tissues was reverse transcribed and subjected to PCR amplification using the following intron-spanning primer sets with the indicated numbers of cycles: PSA PCR primer set (5′-CTTGTGGCCTCTCG-3′ and 5′-GAGGGTGAACTTGC3-′; 35 cycles), mouse β-actin PCR primer set (5′-ATGGATGACGATATCGCTG-3′ and 5′-ATGAGGTAGTCTGTCAGGT-3′; 25 cycles), and human β-actin PCR primer set (5′-GTGGGGCGCCCCAGGCACCA-3′ and 5′-CTCCTTAATGTCACGCACGATTTC-3′; 25 cycles). PSA RT-PCR products were further analyzed by Southern blotting using an internal fragment (nucleotides 158–524) as the probe, which was also labeled by the random hexamer method.
Immunocytochemistry.
Prostate and coagulating gland/seminal vesicle tissue removed from nontransgenic or transgenic mice were fixed in formalin, embedded with paraffin, and sections (5 μm) were placed onto poly-l-lysine-coated slides. Following quenching of endogenous peroxidase, the sections were blocked with normal goat serum, stained with a 1:200 dilution of rabbit anti-human PSA (Dako) followed by goat anti-rabbit Ig conjugated to horse radish peroxidase (Dako), and visualized by adding metal-enhanced diaminobenzidine (Pierce) or 3-amino-9-ethylcarbazole (Vector Laboratories) as the substrate.
TIL Assay.
TIL were purified from tumors grown in transgenic or nontransgenic mice 20 days after injection of 2 × 104 line 1/PSA cells using paramagnetic beads (Dynal, Great Neck, NY) conjugated with anti-Thy-1 monoclonal antibody, as described previously (17). TIL from tumor-bearing mice were assayed for cytotoxic activity in a standard 6-hr assay, against 51Cr-labeled target cells (2,000 per well) at various effector-to-target ratios, as previously described (18).
RESULTS
Generation of Transgenic Mice.
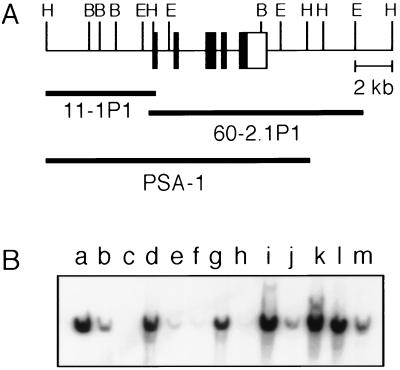
Transgenic mice were generated using the PSA-1 transgene construct, which covers 14 kb of genomic sequence. It contains approximately 6 kb of 5′ flanking sequence and 2 kb of 3′ flanking sequence, in addition to the PSA coding region (Fig. 1A). Of the 94 offspring generated and screened, 9 founders were identified that had incorporated the transgene (Fig. 1B). Of these, six were able to transmit the transgene through their germline, and the transgenic lines P1–2, P1–4, P1–6, P1–7, P1–8, and P1–9 were established. The transgenic lines varied in the number of copies of the transgene they had incorporated, as determined by comparing transgenic bands with copy-number controls of plasmid. High copy-number transgenic lines (approximately 10 copies per diploid genome) included lines P1–4, P1–6, P1–8, and P1–9, whereas P1–2 and P1–7 were low copy-number lines (approximately 1 copy per diploid genome).
Figure 1.
(A) Schematic diagram of the PSA-1 transgene construct. The lambda clones, 60–2.1P1 and 11–1P1, and the assembled PSA-1 transgene construct are depicted in relation to the genomic map. Solid boxes indicate exons, and the open box indicates the 3′ untranslated region of the PSA gene. The 5′ untranslated region is not included because of its small size. (B) Identification of PSA1 transgenic mice. Genomic DNA from mouse tails was used to analyze the incorporation of the PSA-1 transgene construct into the mouse genome. Extracted DNA was digested with BamHI, resolved on a 0.7% agarose gel, transferred to a nylon membrane, and probed with labeled PSA cDNA. Lanes: a–c, normal mouse DNA with an additional equivalent of 10, 1, and 0 copies of the pBS/PSA-1 plasmid per diploid genome to serve as copy number controls; d–l, 9 independent PSA1 transgenic founders designated as P1–1 through P1–9, respectively; m, human hepatoma G2 DNA to serve as a positive control.
Human PSA mRNA Is Expressed in the Prostate of Transgenic Mice.
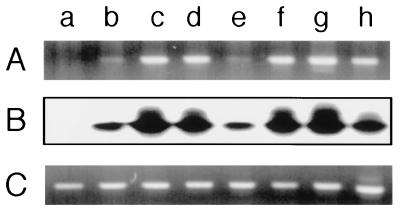
RT-PCR analysis was performed to examine the expression of human PSA transgene in the prostate of the transgenic mice. Total RNA, isolated from mouse prostate and the human prostatic cell line LNCaP, was reverse transcribed into cDNA, which was then subjected to PCR amplification using PSA-specific primers. As shown in Fig. 2A, prostate RNA from all the transgenic lines, as well as RNA from LNCaP, resulted in bands by ethidium bromide staining, whereas prostate RNA from the nontransgenic control did not, indicating that the PSA PCR primers do not cross-react with any mouse gene product. To further ensure that the amplified bands corresponded to PSA, they were probed with a fragment of PSA cDNA that is internal to the RT-PCR product. All the transgenic lines showed hybridization to the internal fragment (Fig. 2B), demonstrating that human PSA mRNA is expressed in the prostate of all the six independent PSA1 transgenic lines. RT-PCR was also performed using mouse and human β-actin primer sets to ensure the integrity of the isolated mouse prostate RNA and LNCaP RNA, respectively (Fig. 2C).
Figure 2.
Expression of PSA transgene in the prostate of the PSA1 transgenics. Total RNA was isolated from the prostate of the nontransgenic and six PSA1 transgenic lines and the human prostatic cell line LNCaP, and RT-PCR was performed with PSA-specific primers and mouse and human β-actin primers. PSA PCR products were visualized by either ethidium bromide staining (A) or Southern blotting using an internal fragment as the probe (B), and β-actin PCR products were visualized by ethidium bromide staining (C). Lanes: a, nontransgenic; b, P1–2; c, P1–4; d, P1–6; e, P1–7; f, P1–8; g, P1–9; h, LNCaP.
Northern Blot Analysis Reveals that Human PSA Has a Highly Restricted Tissue Distribution in PSA Transgenic Mice.
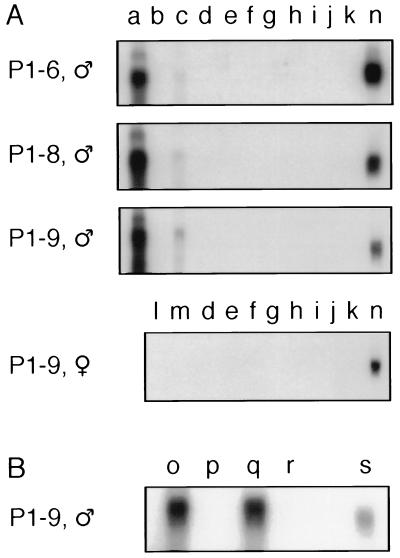
To examine the tissue expression of PSA mRNA, Northern blot analysis was performed on a panel of tissues isolated from males of a nontransgenic control and three independent transgenic lines (P1–6, P1–8, and P1–9). The PSA cDNA probe did not cross-react with any of the mouse kallikrein gene products, because none of the tissues from the nontransgenic control hybridized to the probe (data not shown). In contrast, an intense band of 1.5 kb, corresponding to the major transcript of human PSA mRNA (5, 19), was evident in the prostate of the three PSA1 transgenic lines analyzed and in LNCaP (Fig. 3A). By normalizing to the hybridization intensity of the PSA mRNA in LNCaP, the expression levels of the PSA transgene in P1–6, P1–8, and P1–9 were 0.65-, 1.45-, and 1.65-fold of the level seen in LNCaP, respectively. Furthermore, as with LNCaP and two other human prostate tumor lines (PC 82 and PC EW) (19), four minor alternatively spliced transcripts with the sizes of 5.6, 4.7, 3.2, and 1.9 kb were also observed for the transgene in the prostate of these transgenic mice (data not shown). This indicates that the PSA transgene not only is expressed in the prostate of the transgenics, but also is processed the same way as in humans. The lower molecular weight products, seen in the prostate RNA blot, may represent alternative splicing as well as partial degradation of the RNA.
Figure 3.
Analysis of the tissue distribution of PSA mRNA in the PSA1 transgenics by Northern blotting. (A) Five micrograms of the total RNA isolated from various tissues of a male P1–6, a male P1–8, a male P1–9, and a female P1–9 transgenic mouse were resolved on 1% formaldehyde-agarose gels, transferred to nylon membranes, then hybridized with the labeled PSA cDNA probe. These tissues were: a, prostate; b, testis; c, coagulating gland/seminal vesicle; d, spleen; e, kidney; f, liver; g, thymus; h, heart; i, lung; j, salivary glands; k, brain; l, ovary; m, uterus; and n, 5 μg of LNCaP total RNA in each blot to serve as an internal control. (B) Five μg of the total RNA isolated from prostate (o), testes (p), coagulating gland (q), and seminal vesicle (r) of a male P1–9 transgenic mouse, and 5 μg of LNCaP total RNA (s) were analyzed by Northern blotting with the PSA cDNA probe. Areas between 1 and 2 kb of the blots are shown; hence, only the 1.5-kb major transcript and the 1.9-kb minor alternatively spliced product are visible. The other three longer minor transcripts are not shown.
Even though the Northern blots were intentionally overexposed, the PSA transgene message was not detectable in testis, spleen, kidney, liver, thymus, heart, lung, salivary glands, and brain in any of the three transgenic lines analyzed (Fig. 3A). Significantly, salivary glands and kidney, which are known to express high levels of mouse kallikreins (20, 21), were negative for transgene expression. In addition, the thymus, a critical tissue in the induction of immune tolerance, was also negative. Serial dilution analysis of the LNCaP RNA showed that the Northern blot analysis could detect as little as 0.5% of the PSA expression level seen in LNCaP (data not shown). Because levels of PSA mRNA in the prostate of these transgenics were comparable to that of the LNCaP—if PSA is expressed in any of the nonprostate tissues—their levels must be less than 0.5% of that detected in the prostate. Further Northern blot analysis was also performed on a panel of tissues isolated from a female of the P1–9 transgenic line. No transgene expression was observed in any tissues of the female transgenic mouse, including ovary and uterus (Fig. 3A).
Of interest was the weak but detectable expression in the RNA isolated from tissue composed of the coagulating gland attached to the seminal vesicle, which was not separated in experiments shown in Fig. 3A. To delineate which tissue expresses PSA, coagulating gland from a male P1–9 transgenic mouse was carefully separated from seminal vesicle, and RNA was isolated from each tissue. Northern blot analysis using these separated tissues demonstrated that the PSA transgene is expressed in the coagulating gland but not seminal vesicle of the P1–9 transgenic mouse (Fig. 3B). Based on developmental and anatomical studies, the coagulating gland in rodents has been hypothesized to be analogous to the middle lobe of the human prostate (22), and this model is supported by our data. Taken together, these data show that the human PSA transgene is specifically expressed in the prostate and coagulating gland but is not expressed detectably in other tissues. The highly restricted tissue distribution of the PSA transgene is remarkably similar to the PSA expression pattern reported for humans (5, 23).
PSA Is Expressed in the Epithelial Cells of the Prostate and Coagulating Gland in Transgenic Mice.
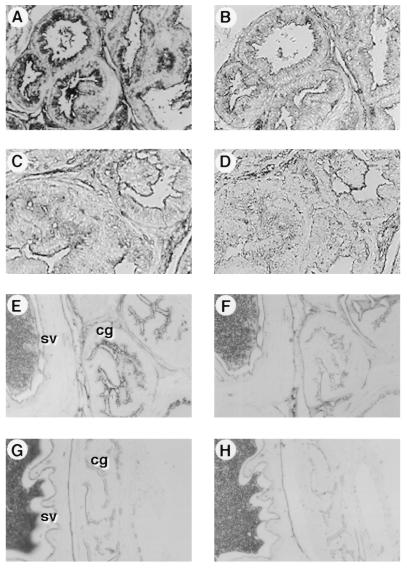
The expression of PSA in the prostate as well as in the coagulating gland was examined by immunocytochemical analysis. Immunohistochemistry using a specific rabbit anti-human PSA antibody demonstrated expression of PSA in the ductal epithelial cells, with the presence of secretory material in the lumen of the prostate gland from the transgenic mice (Fig. 4A). Control sections, in which the primary rabbit anti-PSA antibody was replaced with normal rabbit immunoglobulin, showed little staining (Fig. 4B). This pattern was essentially identical to the staining we had observed on human prostate sections using these reagents (data not shown). Similarly, the epithelial cells of the coagulating gland from the transgenic mice were stained with the specific rabbit anti-human PSA antibody but not with the control antibody (Fig. 4 E and F). On the other hand, in agreement with the Northern blot analysis results, the epithelial cells of the seminal vesicle from the transgenic mice did not show any PSA-specific staining (Fig. 4 E and F). Prostate and coagulating gland/seminal vesicle tissue from nontransgenic mice exhibited no staining above background (Fig. 4 C, D, G, and H). Thus, these results demonstrate that the PSA protein is present in the epithelial cells of the prostate and coagulating gland in the transgenic mice, in a manner reminiscent of the human pattern of expression.
Figure 4.
Localization of PSA expression in the prostate and coagulating gland of the PSA1 transgenics by immunohistochemical staining. Formalin-fixed, paraffin-embedded tissue sections from the prostate (A and B) and coagulating gland/seminal vesicle (E and F) of a P1–9 transgenic, and prostate (C and D) and coagulating gland/seminal vesicle (G and H) of a nontransgenic control were incubated with rabbit anti-human PSA (A, C, E, and G) or control normal rabbit immunoglobulin (B, D, F, and H) followed by HRP-conjugated goat anti-rabbit Ig. Staining was visualized by adding the chromogen diaminobenzidine (A–D) or 3-amino-9-ethylcarbazole (E–H). (E and G) cg, coagulating gland; sv, seminal vesicle.
Transgenic Mice Mount a CTL Response to PSA.
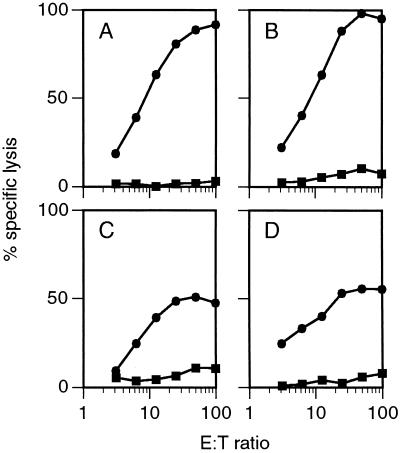
To determine whether the PSA transgenic mice were capable of mounting an immune response to PSA expressed by a tumor, transgenic (line P1–9) and nontransgenic mice were injected with 2 × 104 line 1/PSA cells i.m. in the rear flank. Twenty days later, mice were killed and the tumors removed. TIL were isolated from the tumors, and the ability of these primary CTL to lyse parental line 1 or line 1/PSA tumor cells was evaluated in a 6-hr 51Cr-release assay. Results of two such experiments are illustrated in Fig. 5. The nontransgenic mice were able to mount a vigorous response specific for the PSA antigen, because the PSA-expressing tumor cells were lysed to a high level while the control parental line 1 cells were not lysed (Fig. 5 A and C). The transgenic female mice, being negative for transgene expression, also showed a high level of PSA-specific lysis as expected (data not shown). Remarkably, despite the expression of PSA as a self-antigen in the prostate of the transgenic male mice, they also responded specifically to the PSA-expressing tumor cells (Fig. 5 B and D). It should be noted that previous studies showed that line 1 itself does not induce detectable CTL (17). Although the cytotoxic response appears similar between transgenic and nontransgenic mice, further analysis at the clonal level will be necessary to determine whether the responses are indeed identical in terms of the epitopes responded against and to show formally whether lysis is due to class I-restricted CTL or other effectors. Nevertheless, it appears that these transgenic mice are able to mount a PSA-specific cytolytic response, indicating that expression of the transgene in the prostate has not resulted in complete nonresponsiveness.
Figure 5.
Cytotoxic activity of TIL from line 1/PSA tumors. Nontransgenic (A and C) and transgenic (B and D) mice were injected in the hind flank i.m. with 2 × 104 line 1/PSA cells. Tumors were allowed to grow for 20 days, and TIL were isolated and used as effector cells in a 6-hr 51Cr-release assay. Targets were line 1 (▪) and line 1/PSA (•) induced to express class I with dimethyl sulfoxide as described previously (24). The results of two independent experiments (A and B, C and D) are shown. Because the animals are not inbred, individual mice were analyzed to avoid problems due to alloreactivity that would occur if T cells of different major histocompatibility complex types were mixed together.
DISCUSSION
The PSA-1 transgene construct used in the current studies results in highly tissue-specific expression of the human PSA transgene. Finding the same expression pattern in multiple independent lines of transgenic mice indicates that the tissue-specific expression is independent of the site of integration. This strongly argues that all the necessary information for tissue-specific expression is contained within the PSA-1 construct. In a previous report, a 652-bp fragment of the putative PSA promoter was used to drive the expression of ras oncogene in the transgenic mice (25). This small fragment of the PSA promoter did not confer prostate tissue specificity, nor did it result in expression of ras in prostate (25). In contrast, the experiments presented here demonstrate extremely specific expression of human PSA in the transgenic mice. In addition to the coding region (exons and introns), the PSA-1 construct contains 6 kb of 5′ flanking sequence and 2 kb of 3′ flanking sequence. It is conceivable that the significantly larger 5′ region in the PSA-1 construct contains additional regulatory regions essential for prostate-specific expression. However, it is also possible that the tissue-specific regulatory regions, such as a tissue-specific enhancer or silencer, might be located in one of the introns or in the 3′ flanking region of the PSA-1 construct. This illustrates the strength of the strategy of using the entire gene rather than a small promoter element, particularly when the regulatory elements are largely unknown.
A striking finding is that the regulatory sequences of the human PSA gene function in mice to confer the human expression pattern faithfully. This is particularly surprising because the mouse has approximately 30 kallikrein genes (26), and they are regulated much differently than the human kallikrein, PSA. Indeed, none of the mouse kallikreins identified to date has the same expression pattern as the human PSA (21). Although surprising, this finding is not totally without precedent. Transgenic mice constructed using either the human Thy-1 gene or the human carcinoembryonic antigen gene maintain the human expression patterns of these genes (27, 28). These results suggest that the trans-acting transcription factors are relatively conserved through evolution, and further implies that the cis-acting regulatory DNA sequences are altered to generate different species-specific expression patterns. Thus, this observation may reflect a general principle that would have relevance to targeting genes to specific cell types—either in transgenics or somatically—for gene therapy studies.
Our TIL results demonstrate that it is possible to generate an immune response to PSA in the PSA-expressing transgenic mice. There are many factors that might be expected to contribute to the ability of these mice to respond to PSA. First, PSA is a relatively sequestered antigen. As in humans, only low levels of PSA are expressed in the serum of the male P1–9 transgenics [2.6 ± 0.6 ng/ml (n = 7), as determined by a sandwich ELISA (13)]. In contrast, transgenic mice bearing line 1/PSA tumors express 3- to 6-fold higher levels of PSA in the serum due to PSA expression by the tumors. The local concentration of PSA within the tumor microenvironment is likely to be much higher, which might contribute to the immune response. Second, in humans PSA is androgen regulated, and it is expressed in males after puberty (29). If this is also true in the PSA1 transgenics, it is likely that a large number of PSA-specific T cells have already matured prior to the expression of PSA. Such clones of T cells thus would have escaped deletion in the thymus and would be capable of responding to PSA when presented in an immunogenic form. In addition, immune-mediated prostatitis can be induced in both rats and mice when they are immunized with syngeneic prostate homogenates in the presence of complete Freund’s adjuvant, indicating that it is possible to mount a response to endogenous prostate antigens (30–32). Furthermore, patients with advanced prostate cancer, in which serum PSA levels were greatly increased, also exhibited antibodies to PSA (33). Taken together, these data would suggest that under certain conditions it is possible to engender a response to self-antigens present in the prostate.
There are at least three possible mechanisms of nonresponsiveness to self-antigens that have been proposed (34): clonal deletion, in which self-reactive clones are removed from the repertoire; clonal anergy, in which self-reactive clones are present but functionally inactivated; and clonal ignorance, in which self-reactive clones are present but do not encounter antigens in the proper context for either activation or tolerance induction. Our results are perhaps most consistent with clonal ignorance (35), in that a CTL response can be elicited against PSA by a growing tumor even though it is present as a self-antigen. However, the experiments performed here measured the response against the total protein and did not give information as to which particular epitopes of PSA were recognized. It is possible that the transgenic mice may have deleted clones specific for the dominant epitopes, while clones reactive against other epitopes, either subdominant or cryptic, may still be present. A detailed analysis of the CTL epitopes of PSA in transgenic and nontransgenic mice is required to resolve this issue. In addition, one should be cautious in comparing the quantitative responses in the transgenic and nontransgenic animals due to variability inherent in these analyses, including the differences in the genetic background of these animals. It should also be noted that, although these assays greatly enrich for reactive cells as a result of purifying T cells from the tumors, the tumors were still growing progressively. Hence, the immune response seen in these animals is not sufficient to cause rejection of a highly aggressive tumor at these doses, suggesting that it will be necessary to devise means of enhancing the response for effective therapy. Nevertheless, our results do indicate that expression of PSA in a pattern similar to that of humans does not render these transgenic mice completely tolerant. Therefore, these transgenic mice will provide a well defined system to investigate the relative contribution of clonal deletion, anergy, and ignorance to the nonresponsiveness to PSA.
The PSA1 transgenic mice provide a model system to study the immunological aspects of prostate cancer using authentic human PSA as the tumor antigen. This model not only allows the study of mechanisms of nonresponsiveness to PSA, but has implications for immunotherapy of human prostate cancer as well. For example, immunization strategies for the therapy of prostate cancer using PSA as the target antigen can be tested in these transgenic mice. By crossing these mice to the recently described transgenic mice, which develop cancer in the prostate (36, 37), it will be possible to study the immune response to cancers that develop in situ. Similarly, a detailed analysis of CTL epitope usage in these transgenics will aid in the identification of peptide epitopes of PSA that elicit the most effective anti-tumor response. In this light, crossing these mice to other developed transgenic strains, such as HLA-A2 transgenics (38, 39), will allow development of animal models even more relevant to the treatment of this human disease.
Moreover, the identification of prostate tissue-specific regulatory elements may lead to the construction of specific gene therapy vectors. By incorporating tissue-specific elements into vectors that infect a wide variety of cell types efficiently, cell-type specificity of expression of desired genes can still be maintained. For example, vectors that do not require cells to be actively dividing could, in principle, be used in conjunction with prostate-specific enhancers to drive “suicide” genes, such as thymidine kinase, to confer susceptibility to drugs. Such strategies might be particularly important in relatively slow-growing cancers such as prostate carcinomas.
Acknowledgments
We thank Drs. Jeffrey Frelinger, George Abraham, Nicholas Cohen, and Edward Messing for helpful discussions and encouragement. This work was supported in part by the Edelman-Gardner Foundation, U.S. Public Health Service Grants CA70218 (J.G.F.) and CA28332 (E.M.L.), American Cancer Society Grant IM-569 (R.K.B.), and a grant from the Pepper Center (J.G.F.). C.W. was supported by National Institutes of Health Postdoctoral Training Grant T32CA09363. R.A.W. was supported in part by U.S. Public Health Service Training Grants T32AI07285 and T32GM07102. Transgenic mice were generated in the University of Rochester Cancer Center Transgenic Mouse Facility, which is supported by Cancer Center Core Grant P30CA11198. We thank Dr. Robert Howell and Diana Ramirez for technical assistance in generating the transgenic mice. Genomic DNA from the Hep G2 human hepatoma cell line was provided by Dr. W. Chou, Department of Biochemistry, University of Rochester. Human β-actin PCR primers were provided by Dr. Gregory Sempowski. This is publication number 130 from the Immunology Program of the University of Rochester Cancer Center.
ABBREVIATIONS
- PSA
human prostate-specific antigen
- CTL
cytotoxic T lymphocyte(s)
- TIL
tumor-infiltrating lymphocyte(s)
References
- 1.Parker S L, Tong T, Bolden S, Wingo P A. CA Cancer J Clin. 1996;46:5–27. doi: 10.3322/canjclin.46.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Gittes R F. New Engl J Med. 1991;324:236–245. doi: 10.1056/NEJM199101243240406. [DOI] [PubMed] [Google Scholar]
- 3.Stamey T A, Yang N, Hay A R, McNeal J E, Freiha F S, Redwine E. New Engl J Med. 1987;317:909–916. doi: 10.1056/NEJM198710083171501. [DOI] [PubMed] [Google Scholar]
- 4.Watt K W, Lee P J, M’Timkulu T, Chan W P, Loor R. Proc Natl Acad Sci USA. 1986;83:3166–3170. doi: 10.1073/pnas.83.10.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peehl, D. M. (1995) Cancer 75, Suppl., 2021–2026.
- 6.Brichard V, Van Pel A, Wolfel T, Wolfel C, De Plaen E, Lethe B, Coulie P, Boon T. J Exp Med. 1993;178:489–495. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakker A B, Schreurs M W, de Boer A J, Kawakami Y, Rosenberg S A, Adema G J, Figdor C G. J Exp Med. 1994;179:1005–1009. doi: 10.1084/jem.179.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawakami Y, Eliyahu S, Delgado C H, Robbins P F, Rivoltini L, Topalian S L, Miki T, Rosenberg S A. Proc Natl Acad Sci USA. 1994;91:3515–3519. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang R F, Robbins P F, Kawakami Y, Kang X Q, Rosenberg S A. J Exp Med. 1995;181:799–804. doi: 10.1084/jem.181.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cox A L, Skipper J, Chen Y, Henderson R A, Darrow T L, Shabanowitz J, Engelhard V H, Hunt D F, Slingluff C L., Jr Science. 1994;264:716–719. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- 11.Riegman P H, Vlietstra R J, van der Korput J A, Romijn J C, Trapman J. Biochem Biophys Res Commun. 1989;159:95–102. doi: 10.1016/0006-291x(89)92409-1. [DOI] [PubMed] [Google Scholar]
- 12.Riegman P H, Vlietstra R J, van der Korput J A, Brinkmann A O, Trapman J. Mol Endocrinol. 1991;5:1921–1930. doi: 10.1210/mend-5-12-1921. [DOI] [PubMed] [Google Scholar]
- 13.Wei C, Storozynsky E, McAdam A J, Yeh K-Y, Tilton B R, Willis R A, Barth R K, Looney R J, Lord E M, Frelinger J G. Cancer Immunol Immunother. 1996;42:362–368. doi: 10.1007/s002620050295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dragone L L, Barth R K, Sitar K L, Disbrow G L, Frelinger J G. Proc Natl Acad Sci USA. 1995;92:626–630. doi: 10.1073/pnas.92.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuhas J M, Pazmino N H, Proctor J O, Toya R E. Cancer Res. 1974;34:722–728. [PubMed] [Google Scholar]
- 16.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 17.McAdam A J, Pulaski B A, Storozynsky E, Yeh K-Y, Sickel J Z, Frelinger J G, Lord E M. Cell Immunol. 1995;165:183–192. doi: 10.1006/cimm.1995.1204. [DOI] [PubMed] [Google Scholar]
- 18.Maryanski J L, Van Snick J, Cerottini J C, Boon T. Eur J Immunol. 1982;12:401–406. doi: 10.1002/eji.1830120508. [DOI] [PubMed] [Google Scholar]
- 19.Riegman P H, Klaassen P, van der Korput J A, Romijn J C, Trapman J. Biochem Biophys Res Commun. 1988;155:181–188. doi: 10.1016/s0006-291x(88)81066-0. [DOI] [PubMed] [Google Scholar]
- 20.van Leeuwen B H, Evans B A, Tregear G W, Richards R I. J Biol Chem. 1986;261:5529–5535. [PubMed] [Google Scholar]
- 21.Clements J A. Endocr Rev. 1989;10:393–419. doi: 10.1210/edrv-10-4-393. [DOI] [PubMed] [Google Scholar]
- 22.Price D. Natl Cancer Inst Monogr. 1963;12:1–27. [PubMed] [Google Scholar]
- 23.Clements J A. Mol Cell Endocrinol. 1994;99:C1–C6. doi: 10.1016/0303-7207(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 24.Bahler D W, Lord E M. J Immunol. 1985;134:2790–2798. [PubMed] [Google Scholar]
- 25.Schaffner D L, Barrios R, Shaker M R, Rajagopalan S, Huang S L, Tindall D J, Young C Y, Overbeek P A, Lebovitz R M, Lieberman M W. Lab Invest. 1995;72:283–290. [PubMed] [Google Scholar]
- 26.Evans B A, Drinkwater C C, Richards R I. J Biol Chem. 1987;262:8027–8034. [PubMed] [Google Scholar]
- 27.Gordon J W, Chesa P G, Nishimura H, Rettig W J, Maccari J E, Endo T, Seravalli E, Seki T, Silver J. Cell. 1987;50:445–452. doi: 10.1016/0092-8674(87)90498-3. [DOI] [PubMed] [Google Scholar]
- 28.Eades-Perner A M, van der Putten H, Hirth A, Thompson J, Neumaier M, von Kleist S, Zimmermann W. Cancer Res. 1994;54:4169–4176. [PubMed] [Google Scholar]
- 29.Goldfarb D A, Stein B S, Shamszadeh M, Petersen R O. J Urol. 1986;136:1266–1269. doi: 10.1016/s0022-5347(17)45310-9. [DOI] [PubMed] [Google Scholar]
- 30.Depiante-Depaoli M, Pacheco-Rupil B, Britos S, Casas A. Am J Reprod Immunol. 1984;5:9–14. doi: 10.1111/j.1600-0897.1984.tb00280.x. [DOI] [PubMed] [Google Scholar]
- 31.Pacheco-Rupil B, Depiante-Depaoli M, Casadio B. Am J Reprod Immunol. 1984;5:15–19. doi: 10.1111/j.1600-0897.1984.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 32.Keetch D W, Humphrey P, Ratliff T L. J Urol. 1994;152:247–250. doi: 10.1016/s0022-5347(17)32871-9. [DOI] [PubMed] [Google Scholar]
- 33.Chu T M, Kuriyama M, Johnson E, Papsidero L D, Killian C S, Murphy G P, Wang M C. Transplant Proc. 1984;16:481–485. [PubMed] [Google Scholar]
- 34.Nossal G J. Cell. 1994;76:229–239. doi: 10.1016/0092-8674(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 35.Miller J F A P, Heath W R. Immunol Rev. 1993;133:131–150. doi: 10.1111/j.1600-065x.1993.tb01514.x. [DOI] [PubMed] [Google Scholar]
- 36.Maroulakou I G, Anver M, Garrett L, Green J E. Proc Natl Acad Sci USA. 1994;91:11236–11240. doi: 10.1073/pnas.91.23.11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenberg N M, DeMayo F, Finegold M J, Medina D, Tilley W D, Aspinall J O, Cunha G R, Donjacour A A, Matusik R J, Rosen J M. Proc Natl Acad Sci USA. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le A X, Bernhard E J, Holterman M J, Strub S, Parham P, Lacy E, Engelhard V H. J Immunol. 1989;142:1366–1371. [PubMed] [Google Scholar]
- 39.Vitiello A, Marchesini D, Furze J, Sherman L A, Chesnut R W. J Exp Med. 1991;173:1007–1015. doi: 10.1084/jem.173.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]