Abstract
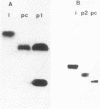
Pentalenolactone (PL), an antibiotic produced by Streptomyces arenae, is a potent inhibitor of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The producer strain contains different isoforms of GAPDH: a PL-sensitive enzyme on nonproduction media and a PL-insensitive enzyme on production media. After induction of PL synthesis, the sensitive GAPDH disappears parallel to the disappearance of its activity, as shown by Western (immunoblot) hybridization. The two isoenzymes exhibit little immunological cross-reactivity and differ in size, amino acid composition, and several amino acid residues of their amino termini. Two different types of plasmids from a S. arenae genomic library, named pBRPLR1 and pBRPLR2, were cloned in Escherichia coli by selection for enhanced PL resistance. Both contain a GAPDH structural gene. Plasmid pBRPLR1 increases E. coli PL tolerance 7-fold, and plasmid pBRPLR2 increases it 30-fold. GAPDH from pBRPLR1 transformants shows biphasic PL inactivation kinetics. These cells contain PL-sensitive GAPDH from both E. coli and S. arenae. GAPDH from pBRPLR2 transformants tolerates higher PL concentrations than either E. coli or S. arenae PL-sensitive GAPDH but is less resistant than S. arenae PL-insensitive GAPDH. Nondenaturing polyacrylamide electrophoresis showed this GAPDH to be a hybrid of E. coli and S. arenae PL-insensitive GAPDH. The hybrid enzyme could be purified to homogeneity. Induction of the lacZ promoter of pUC subclones of both GAPDH genes had only a small effect on raising the level of intracellular GAPDH.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Branlant G., Branlant C. Nucleotide sequence of the Escherichia coli gap gene. Different evolutionary behavior of the NAD+-binding domain and of the catalytic domain of D-glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1985 Jul 1;150(1):61–66. doi: 10.1111/j.1432-1033.1985.tb08988.x. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Carlson C. W., Brosemer R. W. Comparative structural properties of insect triose phosphate dehydrogenases. Biochemistry. 1971 May 25;10(11):2113–2119. doi: 10.1021/bi00787a024. [DOI] [PubMed] [Google Scholar]
- Chater K. F., Hopwood D. A., Kieser T., Thompson C. J. Gene cloning in Streptomyces. Curr Top Microbiol Immunol. 1982;96:69–95. doi: 10.1007/978-3-642-68315-2_5. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Duszenko M., Balla H., Mecke D. Specific inactivation of glucose metabolism from eucaryotic cells by pentalenolactone. Biochim Biophys Acta. 1982 Feb 2;714(2):344–350. doi: 10.1016/0304-4165(82)90343-9. [DOI] [PubMed] [Google Scholar]
- Duszenko M., Mecke D. Inhibition of glyceraldehyde-3-phosphate dehydrogenase by pentalenolactone in Trypanosoma brucei. Mol Biochem Parasitol. 1986 Jun;19(3):223–229. doi: 10.1016/0166-6851(86)90004-6. [DOI] [PubMed] [Google Scholar]
- Entian K. D., Fröhlich K. U., Mecke D. Regulation of enzymes and isoenzymes of carbohydrate metabolism in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 1984 Jun 15;799(2):181–186. doi: 10.1016/0304-4165(84)90293-9. [DOI] [PubMed] [Google Scholar]
- FERGUSON K. A. STARCH-GEL ELECTROPHORESIS--APPLICATION TO THE CLASSIFICATION OF PITUITARY PROTEINS AND POLYPEPTIDES. Metabolism. 1964 Oct;13:SUPPL–SUPPL1002. doi: 10.1016/s0026-0495(64)80018-4. [DOI] [PubMed] [Google Scholar]
- Franke M., Rohrschneider S., Geiger R. Enzyme immunoassay of human urinary kallikrein. Determination of human urinary kallikrein, III. J Clin Chem Clin Biochem. 1982 Sep;20(9):621–626. doi: 10.1515/cclm.1982.20.9.621. [DOI] [PubMed] [Google Scholar]
- Fröhlich K. U., Entian K. D., Mecke D. Cloning and restriction analysis of the hexokinase PII gene of the yeast Saccharomyces cerevisiae. Mol Gen Genet. 1984;194(1-2):144–148. doi: 10.1007/BF00383509. [DOI] [PubMed] [Google Scholar]
- Graser T. A., Godel H. G., Albers S., Földi P., Fürst P. An ultra rapid and sensitive high-performance liquid chromatographic method for determination of tissue and plasma free amino acids. Anal Biochem. 1985 Nov 15;151(1):142–152. doi: 10.1016/0003-2697(85)90064-8. [DOI] [PubMed] [Google Scholar]
- Hartmann S., Neeff J., Heer U., Mecke D. Arenaemycin (pentalenolactone): a specific inhibitor of glycolysis. FEBS Lett. 1978 Sep 15;93(2):339–342. doi: 10.1016/0014-5793(78)81135-1. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Hillman J. D., Fraenkel D. G. Glyceraldehyde 3-phosphate dehydrogenase mutants of Escherichia coli. J Bacteriol. 1975 Jun;122(3):1175–1179. doi: 10.1128/jb.122.3.1175-1179.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking J. D., Harris J. I. D-glyceraldehyde-3-phosphate dehydrogenase. Amino-acid sequence of the enzyme from the extreme thermophile Thermus aquaticus. Eur J Biochem. 1980 Jul;108(2):567–579. doi: 10.1111/j.1432-1033.1980.tb04752.x. [DOI] [PubMed] [Google Scholar]
- Hopwood D. A., Kieser T., Wright H. M., Bibb M. J. Plasmids, recombination and chromosome mapping in Streptomyces lividans 66. J Gen Microbiol. 1983 Jul;129(7):2257–2269. doi: 10.1099/00221287-129-7-2257. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Maurer K. H., Pfeiffer F., Zehender H., Mecke D. Characterization of two glyceraldehyde-3-phosphate dehydrogenase isoenzymes from the pentalenolactone producer Streptomyces arenae. J Bacteriol. 1983 Feb;153(2):930–936. doi: 10.1128/jb.153.2.930-936.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misset O., Van Beeumen J., Lambeir A. M., Van der Meer R., Opperdoes F. R. Glyceraldehyde-phosphate dehydrogenase from Trypanosoma brucei. Comparison of the glycosomal and cytosolic isoenzymes. Eur J Biochem. 1987 Feb 2;162(3):501–507. doi: 10.1111/j.1432-1033.1987.tb10668.x. [DOI] [PubMed] [Google Scholar]
- Nakane P. K., Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974 Dec;22(12):1084–1091. doi: 10.1177/22.12.1084. [DOI] [PubMed] [Google Scholar]
- Nakano M. M., Mashiko H., Ogawara H. Cloning of the kanamycin resistance gene from a kanamycin-producing Streptomyces species. J Bacteriol. 1984 Jan;157(1):79–83. doi: 10.1128/jb.157.1.79-83.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan M. J., Marks V. Methods for the preparation of enzyme-antibody conjugates for use in enzyme immunoassay. Methods Enzymol. 1981;73(Pt B):147–166. doi: 10.1016/0076-6879(81)73062-3. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Harris J. I. Hybridization of glyceraldehyde-3-phosphate dehydrogenase. J Biochem. 1975 Mar;77(3):587–593. doi: 10.1093/oxfordjournals.jbchem.a130760. [DOI] [PubMed] [Google Scholar]
- Thiara A. S., Cundliffe E. Cloning and characterization of a DNA gyrase B gene from Streptomyces sphaeroides that confers resistance to novobiocin. EMBO J. 1988 Jul;7(7):2255–2259. doi: 10.1002/j.1460-2075.1988.tb03065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J., Rae S., Cundliffe E. Coupled transcription--translation in extracts of Streptomyces lividans. Mol Gen Genet. 1984;195(1-2):39–43. doi: 10.1007/BF00332721. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Carne A. F., Runswick M. J., Bridgen J., Harris J. I. D-glyceraldehyde-3-phosphate dehydrogenase. Complete amino-acid sequence of the enzyme from Bacillus stearothermophilus. Eur J Biochem. 1980 Jul;108(2):549–565. doi: 10.1111/j.1432-1033.1980.tb04751.x. [DOI] [PubMed] [Google Scholar]