Abstract
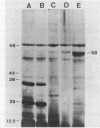
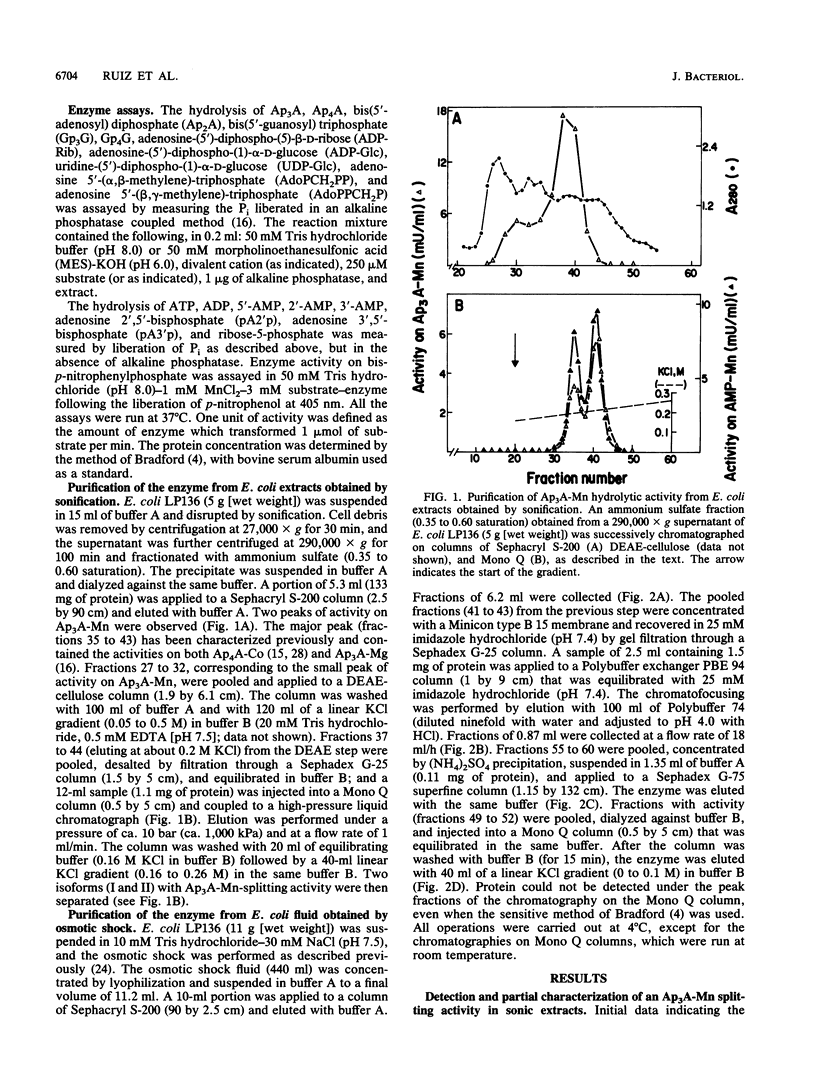
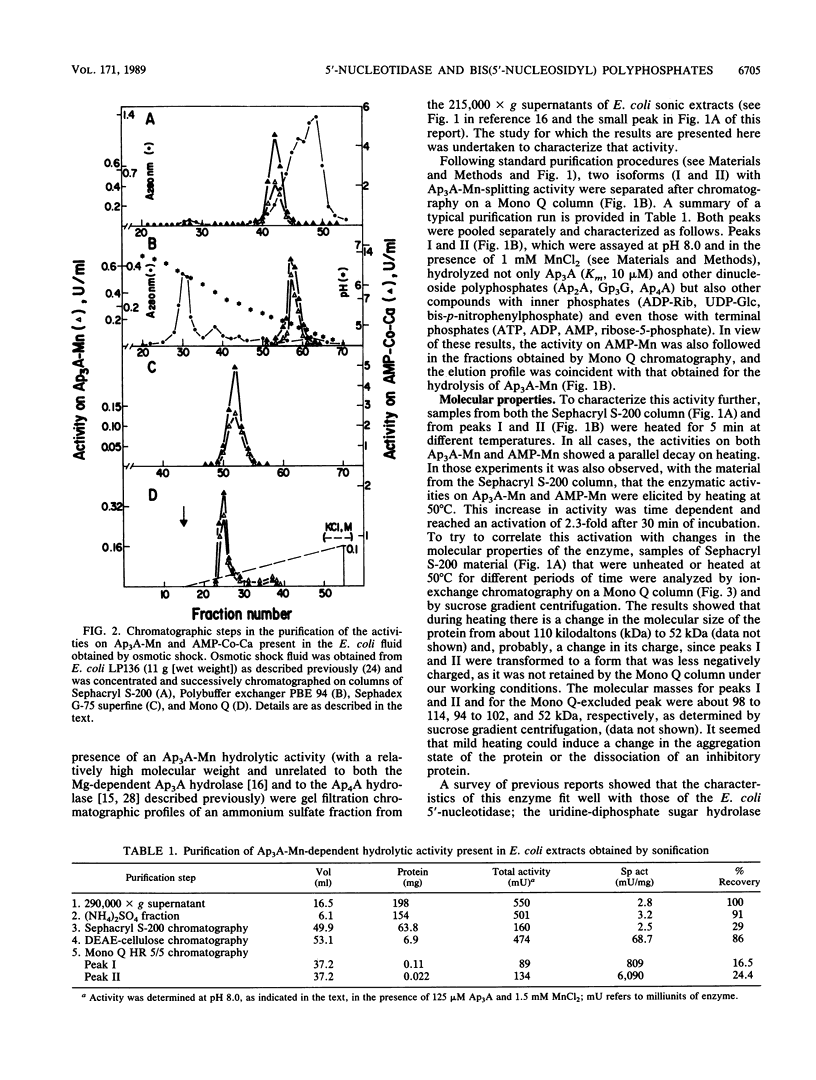
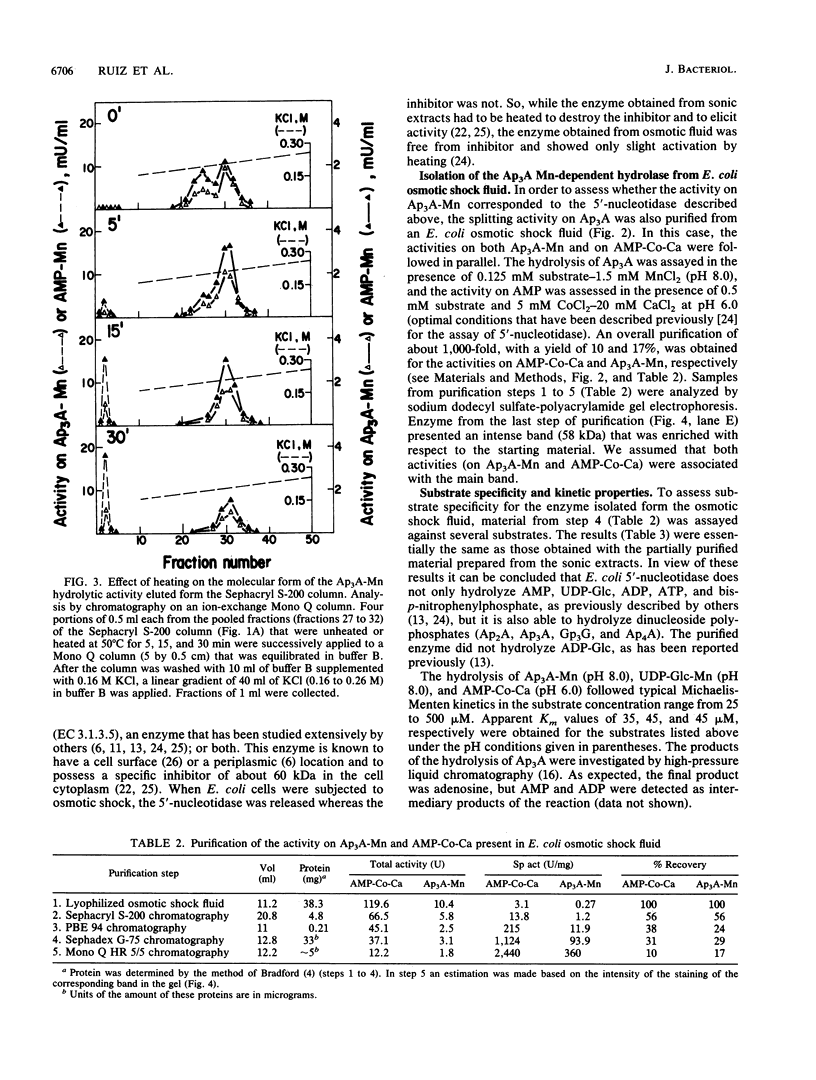
Two enzymatic activities that split diadenosine triphosphate have been reported in Escherichia coli: a specific Mg-dependent bis(5'-adenosyl) triphosphatase (EC 3.6.1.29) and the bis(5'-adenosyl) tetraphosphatase (EC 3.6.1.41). In addition to the activities of these two enzymes, a different enzyme activity that hydrolyzes dinucleoside polyphosphates is described. After purification and study of its molecular and kinetic properties, we concluded that it corresponded to the 5'-nucleotidase (EC 3.1.3.5) that has been described in E. coli. The enzyme was purified from sonic extracts and osmotic shock fluid. From sonic extracts, two isoforms were isolated by chromatography on ion-exchange Mono Q columns; they had a molecular mass of about 100 kilodaltons (kDa). From the osmotic shock fluid, a unique form of 52 kDa was recovered. Mild heating transformed the 100-kDa isoform to a 52-kDa form, with an increase in activity of about threefold. The existence of a 5'-nucleotidase inhibitor described previously, which associates with the enzyme and is not liberated in the osmotic shock fluid, may have been responsible for these results. The kinetic properties and substrate specificities of both forms (52 and 100 kDa) were almost identical. The enzyme, which is known to hydrolyze AMP and uridine-(5')-diphospho-(1)-alpha-D-glucose, but not adenosine-(5')-diphospho-(1)-alpha-D-glucose, was also able to split adenosine-(5')-diphospho-(5)-beta-D-ribose, ribose-5-phosphate, and dinucleoside polyphosphates [diadenosine 5',5'''-P1,P2-diphosphate,diadenosine 5',5'''-P1,P3-triphosphate, diadenosine 5',5'''-P1,P4-tetraphosphate, and bis(5'-guanosyl) triphosphate]. The effects of divalent cations and pH on the rate of the reaction with different substrates were studied.
Full text
PDF
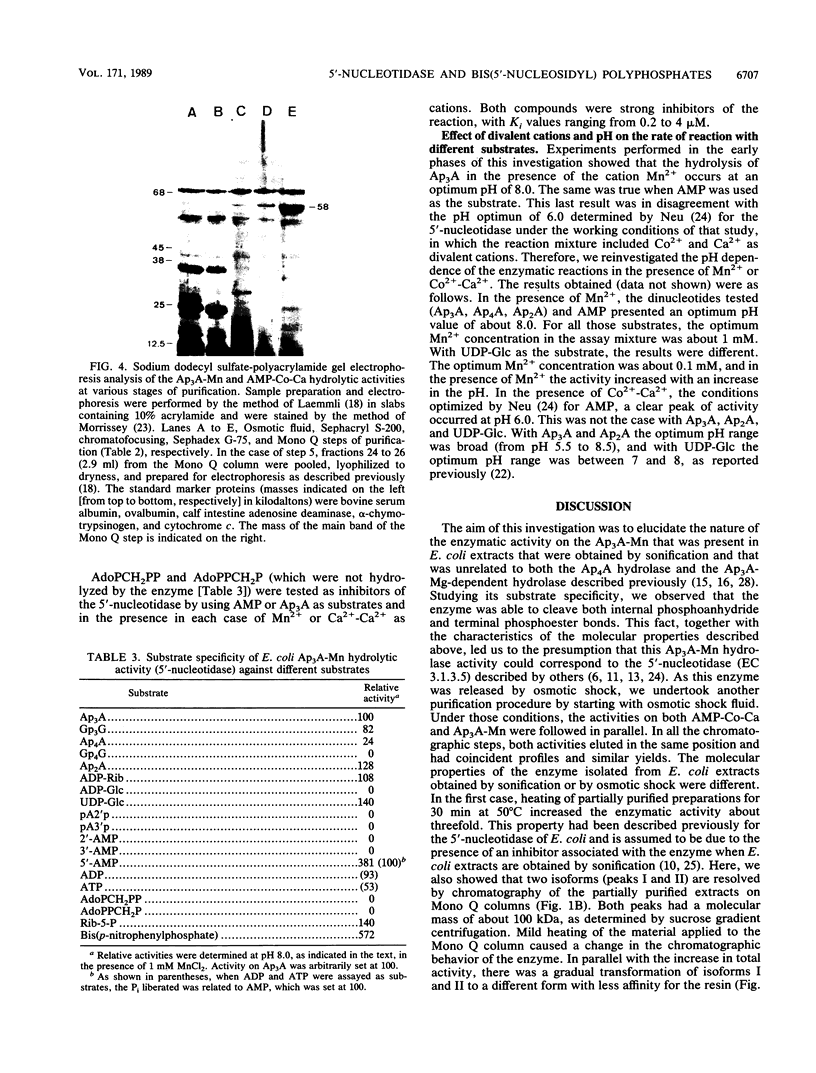


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J. C., Jacobson M. K. Alteration of adenyl dinucleotide metabolism by environmental stress. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2350–2352. doi: 10.1073/pnas.83.8.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchin-Roland S., Blanquet S., Schmitter J. M., Fayat G. The gene for Escherichia coli diadenosine tetraphosphatase is located immediately clockwise to folA and forms an operon with ksgA. Mol Gen Genet. 1986 Dec;205(3):515–522. doi: 10.1007/BF00338091. [DOI] [PubMed] [Google Scholar]
- Bochner B. R., Lee P. C., Wilson S. W., Cutler C. W., Ames B. N. AppppA and related adenylylated nucleotides are synthesized as a consequence of oxidation stress. Cell. 1984 May;37(1):225–232. doi: 10.1016/0092-8674(84)90318-0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brevet A., Plateau P., Best-Belpomme M., Blanquet S. Variation of Ap4A and other dinucleoside polyphosphates in stressed Drosophila cells. J Biol Chem. 1985 Dec 15;260(29):15566–15570. [PubMed] [Google Scholar]
- Broad D. F., Smith J. T. Escherichia coli 5'-nucleotidase: purification, properties and its release by osmotic shock. J Gen Microbiol. 1981 Apr;123(2):241–247. doi: 10.1099/00221287-123-2-241. [DOI] [PubMed] [Google Scholar]
- Cameselle J. C., Costas M. J., Günther Sillero M. A., Sillero A. Two low Km hydrolytic activities on dinucleoside 5',5"'-P1,P4-tetraphosphates in rat liver. Characterization as the specific dinucleoside tetraphosphatase and a phosphodiesterase I-like enzyme. J Biol Chem. 1984 Mar 10;259(5):2879–2885. [PubMed] [Google Scholar]
- Dvorak H. F., Anraku Y., Heppel L. A. The occurrence of a protein inhibitor for 5'-nucleotidase in extracts of Escherichia coli. Biochem Biophys Res Commun. 1966 Sep 8;24(5):628–632. doi: 10.1016/0006-291x(66)90369-x. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F., Heppel L. A. Metallo-enzymes released from Escherichia coli by osmotic shock. II. Evidence that 5'-nucleotidase and cyclic phosphodiesterase are zinc metallo-enzymes. J Biol Chem. 1968 May 25;243(10):2647–2653. [PubMed] [Google Scholar]
- Garrison P. N., Mathis S. A., Barnes L. D. In vivo levels of diadenosine tetraphosphate and adenosine tetraphospho-guanosine in Physarum polycephalum during the cell cycle and oxidative stress. Mol Cell Biol. 1986 Apr;6(4):1179–1186. doi: 10.1128/mcb.6.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser L., Melo A., Paul R. Uridine diphosphate sugar hydrolase. Purification of enzyme and protein inhibitor. J Biol Chem. 1967 Apr 25;242(8):1944–1954. [PubMed] [Google Scholar]
- Guranowski A., Jakubowski H., Holler E. Catabolism of diadenosine 5',5"'-P1,P4-tetraphosphate in procaryotes. Purification and properties of diadenosine 5',5"'-P1,P4-tetraphosphate (symmetrical) pyrophosphohydrolase from Escherichia coli K12. J Biol Chem. 1983 Dec 25;258(24):14784–14789. [PubMed] [Google Scholar]
- Hurtado C., Ruíz A., Sillero A., Sillero M. A. Specific magnesium-dependent diadenosine 5',5'''-P1,P3-triphosphate pyrophosphohydrolase in Escherichia coli. J Bacteriol. 1987 Apr;169(4):1718–1723. doi: 10.1128/jb.169.4.1718-1723.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski H., Guranowski A. Enzymes hydrolyzing ApppA and/or AppppA in higher plants. Purification and some properties of diadenosine triphosphatase, diadenosine tetraphosphatase, and phosphodiesterase from yellow lupin (Lupinus luteus) seeds. J Biol Chem. 1983 Aug 25;258(16):9982–9989. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee P. C., Bochner B. R., Ames B. N. AppppA, heat-shock stress, and cell oxidation. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7496–7500. doi: 10.1073/pnas.80.24.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P. C., Bochner B. R., Ames B. N. Diadenosine 5',5"'-P1,P4-tetraphosphate and related adenylylated nucleotides in Salmonella typhimurium. J Biol Chem. 1983 Jun 10;258(11):6827–6834. [PubMed] [Google Scholar]
- Mechulam Y., Fromant M., Mellot P., Plateau P., Blanchin-Roland S., Fayat G., Blanquet S. Molecular cloning of the Escherichia coli gene for diadenosine 5',5'''-P1,P4-tetraphosphate pyrophosphohydrolase. J Bacteriol. 1985 Oct;164(1):63–69. doi: 10.1128/jb.164.1.63-69.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo A., Glaser L. Nucleotide diphosphate hexose pyrophosphatases. Biochem Biophys Res Commun. 1966 Mar 8;22(5):524–531. doi: 10.1016/0006-291x(66)90306-8. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The 5'-nucleotidase of Escherichia coli. I. Purification and properties. J Biol Chem. 1967 Sep 10;242(17):3896–3904. [PubMed] [Google Scholar]
- Neu H. C. The 5'-nucleotidase of Escherichia coli. II. Surface localization and purification of the Escherichia coli 5'-nucleotidase inhibitor. J Biol Chem. 1967 Sep 10;242(17):3905–3911. [PubMed] [Google Scholar]
- Nisonson I., Tannenbaum M., Neu H. C. Surface localization of Escherichia coli 5'-nucleotidase by electron microscopy. J Bacteriol. 1969 Nov;100(2):1083–1090. doi: 10.1128/jb.100.2.1083-1090.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie A., Antl W. Diadenosine tetraphosphatase from human leukemia cells. Purification to homogeneity and partial characterization. J Biol Chem. 1983 Apr 10;258(7):4105–4109. [PubMed] [Google Scholar]
- Plateau P., Fromant M., Brevet A., Gesquière A., Blanquet S. Catabolism of bis(5'-nucleosidyl) oligophosphates in Escherichia coli: metal requirements and substrate specificity of homogeneous diadenosine-5',5'''-P1,P4-tetraphosphate pyrophosphohydrolase. Biochemistry. 1985 Feb 12;24(4):914–922. doi: 10.1021/bi00325a016. [DOI] [PubMed] [Google Scholar]
- Robinson A. K., Barnes L. D. Three diadenosine 5',5''-P1,P4-tetraphosphate hydrolytic enzymes from Physarum polycephalum with differential effects by calcium: a specific dinucleoside polyphosphate pyrophosphohydrolase, a nucleotide pyrophosphatase, and a phosphodiesterase. Arch Biochem Biophys. 1986 Aug 1;248(2):502–515. doi: 10.1016/0003-9861(86)90503-5. [DOI] [PubMed] [Google Scholar]
- Sillero A., Ribeiro J. M. Isoelectric points of proteins: theoretical determination. Anal Biochem. 1989 Jun;179(2):319–325. doi: 10.1016/0003-2697(89)90136-x. [DOI] [PubMed] [Google Scholar]
- Sillero M. A., Villalba R., Moreno A., Quintanilla M., Lobatón C. D., Sillero A. Dinucleosidetriphosphatase from rat liver. Purification and properties. Eur J Biochem. 1977 Jun 15;76(2):331–337. doi: 10.1111/j.1432-1033.1977.tb11600.x. [DOI] [PubMed] [Google Scholar]
- Taylor N. S., Beacham I. R. Synthesis and localisation of Escherichia coli UDP-glucose hydrolase (5'-nucleotidase), and demonstration of a cytoplasmic inhibitor of this enzyme in Salmonella typhimurium. Biochim Biophys Acta. 1975 Dec 5;411(2):216–221. doi: 10.1016/0304-4165(75)90301-3. [DOI] [PubMed] [Google Scholar]
- Vallejo C. G., Sillero M. A., Sillero A. Diguanosinetetraphosphate guanylohydrolase in Artemia salina. Biochim Biophys Acta. 1974 Jul 17;358(1):117–125. doi: 10.1016/0005-2744(74)90264-2. [DOI] [PubMed] [Google Scholar]
- Zamecnik P. Diadenosine 5',5"'-P1,P4-tetraphosphate (Ap4A): its role in cellular metabolism. Anal Biochem. 1983 Oct 1;134(1):1–10. doi: 10.1016/0003-2697(83)90255-5. [DOI] [PubMed] [Google Scholar]