Abstract
The expression of cyclin-dependent kinase inhibitor p27kip1 in human tumors and normal tissues was investigated using a panel of novel anti-p27kip1 mAbs. An inverse correlation between expression of p27kip1 and cell proliferation was generally observed after analyzing its expression in 25 different normal human tissues. In some highly proliferative human breast cancer cells, however, high level p27kip1 expression was seen, indicating the existence of a mechanism by which some growing tumor cells may tolerate this inhibitor of cell cycle progression. Detailed studies demonstrated a correlation between the high level expression of p27kip1 and cyclin D1 in human breast cancer cells. There was also an inverse correlation between the expression of p27kip1 and the degree of tumor malignancy in human breast and colorectal cancers, indicating that p27kip1 may be a useful prognostic marker in these cancers.
In mammalian cells, the G1/S transition is believed to be regulated by complexes of cyclin E/cdk2 and cyclin D/cdk4 or cdk6; the kinase activity of these cdks requires association between a catalytic subunit (the cdk) and a regulatory subunit (the cyclin) (1, 2). Interestingly, the kinase activity of cdks can be inhibited by various kinase inhibitors. There are two families of cdk inhibitors, the cip/kip and ink4 families. The proteins p21waf1/cip1, p27kip1, and p57kip2 belong to the cip/kip family while the ink4 family consists of p16ink4A, p15ink4B, p18ink4C, and p19ink4D. Members of the cip1/kip1 family share sequence homology and can inhibit the kinase activity of a variety of cdks. In contrast, the members of the ink4 family can specifically inhibit the kinase activity of ckd4/ckd6. All cdk inhibitors cause G1 arrest when overexpressed in transfected cells.
The existing evidence suggests that p27kip1 mediates G1 arrest induced by transforming growth factor β, rapamycin, cAMP, contact inhibition, or serum deprivation (3–7). Down-regulation of p27kip1 expression has been seen in interleukin 2-induced T cell proliferation, indicating that p27kip1 may play an important role in the negative regulation of cell growth (6, 8). The development of multiple organ hyperplasia and pituitary tumors in p27kip1 knock out mice demonstrated that p27kip1 plays an important role in repressing tumor development (9–11).
Mutation of p27kip1 has been investigated in a large variety of human tumors using Southern blot analysis, PCR/SSCP, and direct sequencing. Among the 30 primary human breast carcinomas studied, no mutation in the p27kip1 gene was found (12). Deletion, rearrangement, and mutation of the p27kip1 gene has also been shown to be a rare event as a result of studies of over 500 cases of human cancers and 20 different tumor cell lines (13, 14). Alterations of the p27kip1 gene were only seen rarely in a large number of lymphomas and leukaemia studied (15, 16). Taken together, all these data suggest that, unlike p16ink4, mutation in p27kip1 is a rare event in human cancers. Since the main function of p27kip1 is its ability to suppress cell growth, it is possible that during tumor development, to overcome the growth inhibitory activity of p27kip1, tumor cells can down-regulate the protein expression of p27kip1. Recent studies have demonstrated that p27kip1 level is mainly regulated at posttranscriptional levels through protein translation and degradation (17, 18). To study the role p27kip1 may play in the development of human tumors, a panel of monoclonal anti-p27kip1 antibodies was generated. The expression of p27kip1 was analyzed in 25 different human normal tissues. An inverse correlation between p27kip1 expression and cell proliferation was generally seen. However, this inverse correlation was not always observed in the human tumor cells studied. High level expression of p27kip1 was seen in a number of highly proliferative human breast cancer cell lines in vitro. Such high levels of p27kip1 expression were also seen in a large number of tumor cells in vivo. Interestingly, the high level expression of p27kip1 is accompanied by overexpression of cyclin D1 both in vitro and in vivo in human breast cancer cells. Finally, an inverse correlation between the expression of p27kip1 and the degree of tumor malignancy was observed in human breast and colorectal tumors.
MATERIALS AND METHODS
Production of mAb.
The coding region of human p27kip1 was amplified by PCR using Pfu polymerase (Stratagene). The product was cloned into pGEX-2T (Pharmacia) at the BamHI site. A 50-kDa glutathione S-transferase fusion protein (GST)–p27kip1 was produced and used to immunize BALB/c mice (50 μg/mouse). The hybridoma fusion was carried out as described (19). Among the 200 positive clones, two (SX18F7 and SX53G8) were found to have the highest affinity for p27kip1 protein.
Cells and Antibodies.
Human breast cancer cell lines were grown in 50% DMEM and 50% F12-Ham supplemented with 10% fetal calf serum. Human primary myoepithelial and luminal epithelial cells were separated as described using the macs system (20). mAbs SX18F7 and SX53G8 are described in this paper. Anti-cyclin D1 antibody DCS-6 was purchased from Vector Laboratories (21). Rabbit polyclonal anti-cyclin D1 and cdk4 antibodies were a gift from G. Peters (Imperial Cancer Research Fund) and anti-cyclin E and anti-cdk2 antibodies were purchased from Santa Cruz Biotechnology.
Immunoblotting.
Cells were lysed with Nonidet P-40 lysis buffer as described (22). For immunoblotting, 15 μl of soluble cellular proteins (10–15 mg/ml of total cellular protein) were loaded on SDS/polyacrylamide gels in the presence of SDS sample buffer. Primary antibody was added to the blot and incubated for 2–3 h at room temperature or 4°C overnight. The peroxidase-conjugated secondary antibody was incubated with the membrane and proteins detected by the enhanced chemiluminescence method (Amersham).
RNase Protection Assay.
p27kip1 mRNA was detected by RNase protection assay using the RPA II kit (Ambion, Austin, TX). A 32P-labeled antisense riboprobe complementary to p27kip1 RNA nucleotides 340–597 was generated using EcoRI-linearized plasmid pBSK-p27kip1 and T3 RNA polymerase. Total RNA (20 μg) and p27kip1 riboprobe (50,000 Cerenkov cpm) were used in the assay. Yeast RNA (20 μg) was used as a negative control.
Immunohistochemistry.
Immunohistochemistry was performed as described (19, 23, 24). The immunostaining was performed using primary antibody at a dilution of 1 in 5 of supernatant and overnight incubation at 4°C. Specific binding was detected with an ABC peroxidase system using diaminobenzidine as substrate. Sections were then counterstained with haemotoxylin.
RESULTS AND DISCUSSION
Inverse Correlation Between the Expression of p27kip1 and Cell Proliferation in Normal Human Tissues.
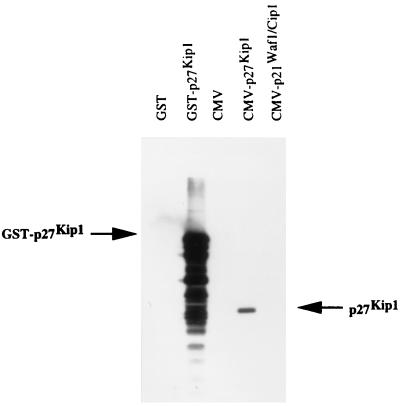
To understand the function of p27kip1 and its role in the development of human tumors, it is important to establish the expression pattern of p27kip1 in normal human tissues. Mice were immunized with GST–p27kip1, and a panel of anti-p27kip1 mAbs was generated and characterized. Two of these anti-p27kip1-specific mAbs, SX18F7 and SX53G8, can specifically recognize p27kip1 through immunoblotting and immunoprecipitation. The specificity of the two anti-p27kip1 antibodies in immunohistochemistry was also demonstrated by depleting these two antibodies with GST–p27kip1 (data not shown). The specificity of SX18F7 is shown in Fig. 1 as an example. Using mAb SX18F7, the p27kip1 expression in 25 different human tissues was examined. Nucleus staining was seen in a wide range of tissues (Table 1). In general, p27kip1 expression was associated with the nonproliferative compartments of tissues such as skeletal muscle, cartilage, smooth muscle, quiescent lymphoid populations, and mesenchymal fibroblasts. Little immunoreactivity was seen in the proliferative compartments of epithelia and only scattered cells showed p27kip1 expression in germinal centers of lymph node. The inverse correlation between the expression of p27kip1 and cell proliferation is a general phenomenon among most of the tissues examined. The inverse correlation between p27kip1 expression and cell proliferation is much tighter than that seen with p21waf1/cip1 (19). This is in agreement with the results obtained from mice in which the p27kip1 or p21waf1/cip1 genes have been knocked out. Multiorgan hyperplasia, pituitary tumors, and increased body size were seen in mice lacking p27kip1 (9–11) but not in mice lacking p21waf1/cip1 (25). All these points argue strongly that p27kip1 is essential in controlling cell proliferation in many different tissues.
Figure 1.
Immunoblotting to show the specificity of anti-p27kip1 mAb SX18F7. SX18F7 can specifically recognize the 50-kDa GST-p27kip1 fusion protein (indicated by arrow GST–p27kip1) but not GST. In transfected Saos-2 cells, SX18F7 can specifically react with 27-kDa p27kip1 (indicated by arrow p27kip1) expressed from CMV–p27kip1 plasmid. It does not crossreact with p21waf1/cip1 overexpressed from CMV–p21waf1/cip1 plasmid.
Table 1.
p27Kip1 expression in normal human tissues
| Tissue | Staining |
|---|---|
| Skin | Labeling in all adnexal structures, including eccrine and sebaceous glands; in epidermis there is labeling in most supra basal cells with increasing intensity as maturation increases; occasional dermal fibroblasts show nuclear staining. |
| Smooth muscle | Occasional cells stain (gut, erector pili, and around vessels) |
| Blood vessels | Smooth muscle and most endothelial cells |
| Lymph node | Many lymphocytes and macrophages in paracortical (T cell areas); occasional cells in germinal centers stain (this is a highly proliferative area, but usually some cells are not cycling); the mantle zone is moderately stained |
| Spleen | As lymph node with many cells in both white and red pulp staining |
| Brain | Almost all glial cells stain but neurones do not |
| Stomach | Occasional cells show nuclear staining but there is no clearly defined pattern seen |
| Small intestine | Occasional stromal cells in the lamina propria stain; on the epithelium there are occasional cells labeling and most are in the differentiated non proliferative populations of the villus |
| Colon | Occasional stromal cells in the lamina propria stain; on the epithelium there are occasional cells labeling |
| Liver | Scattered hepatocytes and Kuppfer cells |
| Gall bladder | Scattered epithelial cells and most stromal cells |
| Pancreas | Islets of Langerhans are stained and occasional acinar cells; rare duct cells stain |
| Salivary gland | Most acinar cells and very occasional duct cells |
| Lung | Cartilage cells and other stromal cells stain; occasional epithelial cell |
| Thyroid | Nearly all cells stain in follicles |
| Breast | Pre- and postmenopausal normal breast very similar; most myo-epithelial cells stain and occasional duct and acinar cells |
| Cervix | Similar to skin (see above) |
| Endometrium | Proliferative phase: occasional glandular cells and most stromal cells; secretory phase: infrequent glandular cells, most stromal cells |
| Fallopian tube | Many epithelial cells and most stromal and smooth muscle cells |
| Ovary | Occasional stromal cells and most follicle lining cells |
| Testis | Spermatogonia and Sertoli cells stain |
| Vas deferens | Only smooth muscle, not epithelium |
| Prostate | Most myoepithelial cells and glandular epithelium as well as most stromal cells |
| Kidney | No tubules stain but scattered cells in mesangium and glomerulus |
| Bladder | Similar to stratified squamous epithelium with increased staining in superficial layers |
High Level Expression of p27kip1 in Highly Proliferative Human Breast Cancer Cell Lines.
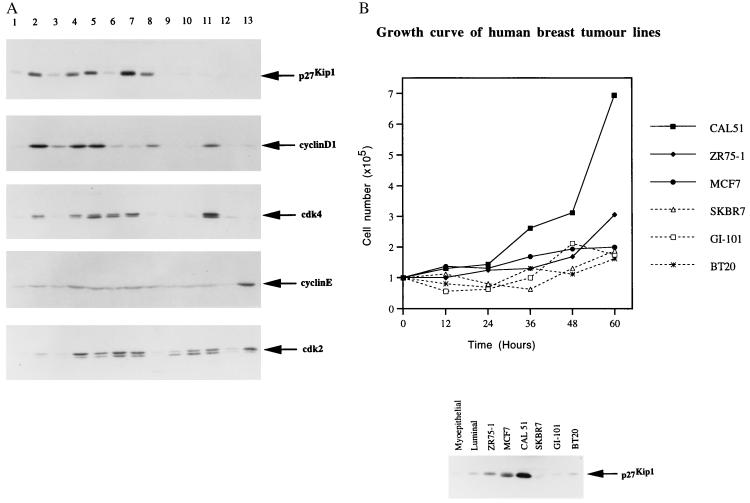
The p27kip1 expression in normal human tissues described above suggests that down-regulation of p27kip1 might be accompanied by an increase in cell proliferation. Such low p27kip1 expression might be expected in human tumors. Expression of p27kip1 was first investigated in 12 different human breast cancer cell lines. High level p27kip1 expression was seen in 5 of the 12 human breast cancer cell lines tested (Fig. 2A). Interestingly, by measuring the growth rate of six representative cell lines (ZR25-1, MCF-7, and CAL51 express p27kip1 at high level but SKBR7, GI101, and BT20 express p27kip1 at low level), it is clear that there is no correlation between the expression level of p27kip1 and the proliferation rate of the cells (Fig. 2B). These results demonstrated that some of the rapidly proliferating human tumor cell lines can tolerate high levels of p27kip1. High level expression of p27kip1 has also recently been seen in proliferating thyroid epithelial cells (26).
Figure 2.
(A) Immunoblotting analysis of p27kip1, cyclin D1, cdk4, cyclin E, and cdk2 expression in human breast cancer cell lines as indicated. The anti-p27kip1 mAb SX53G8 and anti-cyclin D1, anti-cdk4, anti-cyclin E, and anti-cdk2 rabbit polyclonal antibodies are described in Materials and Methods. Lane 1 is the extract from early passaged primary breast myoepithelial cells. Lanes 2–13 are the human breast tumor cell lines: ZR75-1, ZR75-30, MCF-7, MDAMB453, T47D, CAL51, 734B, SKBR5, SKBR7, CAMA, GI-101, and BT20, respectively. (B) Growth curve of six human breast cancer cell lines as indicated (Upper, CAL51, ZR75-1, MCF-7, SKBR7, GI-101, and BT20). (Lower) The p27kip1 protein level of the same group of human breast cancer cell lines is shown. The p27kip1 expression level in the normal primary myoepithelial and luminal epithelial cells is used as a normal control and is labeled as myoepithelial and luminal, respectively.
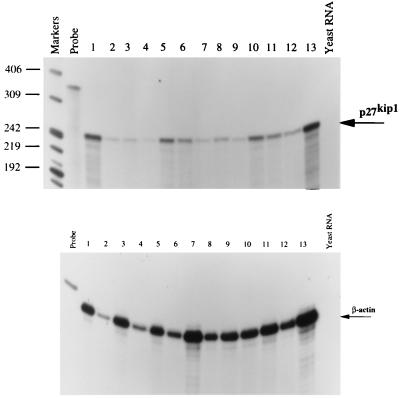
To investigate the level at which p27kip1 expression is regulated, the mRNA for p27kip1 in the 12 breast cancer cell lines described above was analyzed by RNase protection assay. A small variation in p27kip1 mRNA level was detected but there was no direct correlation between the expression levels of p27kip1 protein and mRNA in these cell lines (Figs. 2A and Fig. 3). This result suggests that the high level of p27kip1 expressed in the human breast cancer cell lines is controlled at a posttranscriptional level. It has recently been shown that p27kip1 expression can be controlled by its translation as well as degradation (17, 18). Whether such mechanisms are responsible for the high levels expression of p27kip1 in the tumor cells studied here is currently under investigation.
Correlation Between the High Level Expression of p27kip1 and Cyclin D1 in Human Breast Cancer Cells.
Since high level p27kip1 expression can be seen in highly proliferative breast cancer cell lines such as MCF-7, these breast cancer cell lines must have some other mechanism which allows them to tolerate the high levels p27kip1 expression. p27kip1 was originally isolated through its interaction with cyclin D and cdk4. As an universal inhibitor of cdks, p27kip1 can inhibit the kinase activities of cyclin D/cdk4 and cyclin E/cdk2. It is therefore possible that overexpression of cyclin D/cdk4 or cyclin E/cdk2 might overcome the inhibition of p27kip1 and allow cells to proliferate. It would be the balance of the two opposing signals, p27kip1 and cyclins/cdks, rather than the absolute level of the individual signals which would determine the proliferation status of the cells. This hypothesis was tested by investigating the expression level of cyclin D1, cdk4, cyclin E, and cdk2 relative to p27kip1 in the 12 human breast cancer cell lines used above (Fig. 2A). As shown in Fig. 2A, most of the human breast cancer cell lines expressing high levels of p27kip1 contain cyclin D1 at a higher level (Fig. 2A, lanes 2, 4, and 5). Interestingly, some of the cells expressing high level p27kip1 also expressed a relatively higher level of cdk4 (Fig. 2A, lanes 2 and 4–7). Unlike that of cyclin D1 and cdk4, the expression level of cyclin E is very much the same across all the cell lines (Fig. 2A). There was also no correlation between the expression level of cdk2 and p27kip1 in the 12 human breast cancer cell lines (Fig. 2A). There may thus be a correlation between the expression level of p27kip1 and cyclin D1 or cdk4 which could be one of the mechanisms through which p27kip1 was tolerated at high levels in these cells.
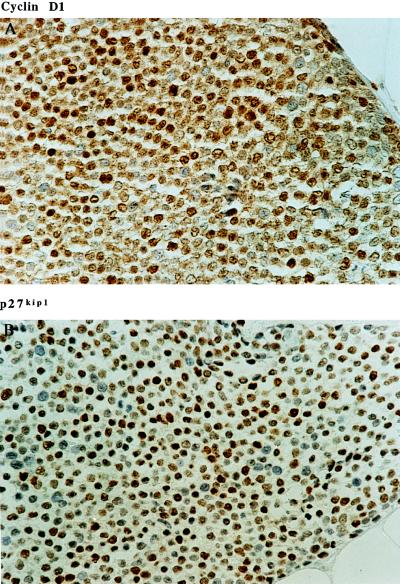
High level expression of cyclin D1 has been seen in a large number of human breast tumors. To see whether the association of cyclin D1 and p27kip1 overexpression seen in the breast cancer cell lines also exists in vivo, sections from 63 human breast tumors with known cyclin D1 expression status were used to investigate their p27kip1 expression (24). As seen in the breast cancer cell lines, nearly all of the tumors that overexpress cyclin D1 (31 out of 33) showed high level expression of p27kip1 (Table 2). The statistical analysis shows that the in vivo correlation between cyclin D1 and p27kip1 expression is a highly significant association (χ2 10.07, P ≪ 0.002). An example of one of such tumor demonstrating cyclin D1 and p27kip1 overexpression is shown in Fig. 4.
Table 2.
Expression of cyclin D1 and p27kip1 in human breast cancers
| p27kip1 | Staining | + | − | Total |
|---|---|---|---|---|
| Cyclin D1 | (+) | 31 | 2 | 33 |
| Staining | (−) | 17 | 13 | 30 |
| Total | 48 | 15 | 63 |
The expression of p27kip1 and cyclin D1 in human breast tumors (63 cases used in a previous study) (24). The numbers of tumors positive and negative for nuclear p27kip1 staining are shown under + or −, respectively.
Figure 4.
Immunohistochemistry staining of cyclin D1 and p27kip1 in the same breast invasive ductal carcinoma using anti-cyclin D1 and anti-p27kip1 mAb DCS-6 and SX53G8 respectively. (×400.)
The correlation between the high level expression of p27kip1 and its opposing partner cyclin D1/cdk4 led us to hypothesize that the true determining factor for cell proliferation is the balance between the two opposing regulators of cell proliferation, p27kip1 and cyclin D1/cdk4. It remains uncertain whether there is a correlation between the expression of p27kip1 and cdk4 but the correlation between the expression levels of p27kip1 and cyclin D1 is clear, though by no means absolute. There are exceptional cell lines and tumor cases in which p27kip1 or cyclin D1 was expressed at high level on its own. For instance, the highest level of p27kip1 was detected in the breast cancer cell line CAL51, but its cyclin D1 level is as low as that seen in the primary epithelial cells. In the cell line CAMA, the opposite was seen; p27kip1 is expressed at low level yet the expression level of cyclin D1 and cdk4 is exceptionally high. Similarly, there are certain primary breast cancer cases which express p27kip1 at high level but the cyclin D1 expression remains low. The factors that are responsible for such discrepancies and the mechanisms that allow the overexpression of p27kip1 and cyclin D1 in these breast tumor cell lines are not yet known. Overexpressing cyclin D1 has recently been shown to induce p27kip1 expression in mouse mammary epithelial cells (27). Thus it is possible that high levels of p27kip1 seen in the human breast tumors were the consequence of overexpression of cyclin D1, but it might also be that high level p27kip1 expression can induce cyclin D1 overexpression. It has been shown previously that in response to transforming growth factor β the decrease in p27kip1/cyclin D/cdk4 complex is accompanied by an increase in the p27kip1/cyclin E/cdk2 complex, suggesting cyclin D/cdk4 can act as a buffer to regulate the precise balance between the p27kip1 and cyclin E/cdk2 (5). If increased p27kip1 expression is one of the cellular responses to proliferation signals, some tumor cells could overcome such inhibition by overexpressing cyclin D1/cdk4. With an increase in malignancy, tumor cells acquire other mechanisms that allow them to down-regulate the expression of inhibitors such as p27kip1 and p21waf1/cip1. Some tumor cells can down-regulate the expression of p21Waf1/cip1 through mutations in the tumor suppressor p53 but the pathways through which tumor cells may down-regulate the expression of p27kip1 remain unknown. However, the ability to repress the activity of p27kip1 could be one of the most important steps in tumor progression. Once the p27kip1 expression level is down-regulated, overexpression of cyclin D1 may no longer be needed. This might explain why high levels of p27kip1 and cyclin D1 were seen in the human breast cancers with lower grade and the absence of p27kip1 and cyclin D1 expression in highly malignant breast tumors with higher grade. Whether or not this is a mechanism specific for human breast cancer is currently under investigation.
Inverse Correlation Between the Expression of Nuclear p27kip1 and the Degree of Malignancy in Human Breast and Colorectal Tumors.
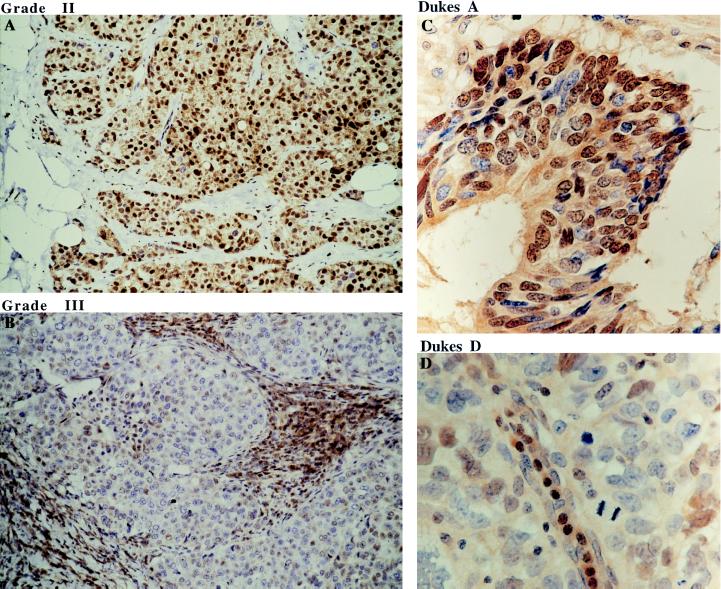
Cyclin D1 has been proposed previously as a prognostic marker in human breast cancer (23). Since there is a good correlation between the expression of cyclin D1 and p27kip1, it was of great interest to study the relationship between the expression of p27kip1 and tumor malignancy. p27kip1 expression was investigated in 84 human breast cancer cases ranging from grade I to grade III. The cellular distribution of p27kip1 in a representative series of breast tumors is shown in Fig. 5. The strong nuclear p27kip1 expression is shown in a grade II human breast tumor (Fig. 5A). In contrast, no nuclear p27kip1 staining was seen in the grade III human breast tumor (Fig. 5B). The expression level of p27kip1 and the degree of malignancy of the tumors tested is summarized in Table 3. High levels of nuclear p27kip1 expression were seen in 92% of grade I (14/15), 80.5% of grade II (29/36), but only 30% of grade III (10/33) human breast tumors. There was a highly significant association between the expression of p27kip1 and the grade of the infiltrating ductal carcinoma (χ2 25.87, P ≪ 0.00001). This result is in agreement with the expression pattern of cyclin D1; the overexpression of cyclin D1 was seen predominantly in the human breast tumors of lower grade (23).
Figure 5.
Immunohistochemistry staining of p27kip1 in human breast and colorectal tumors using anti-p27kip1 mAb SX53G8. (A) Strong nuclear staining of p27kip1 in majority of the tumor cells in grade II invasive ductal carcinoma. (B) In grade III invasive ductal carcinoma, tumor cells are negative for p27kip1, while stromal lymphocytes are strongly stained. (C and D) Nuclear p27kip1 expression is also seen in Dukes A but not Dukes D human colorectal tumors. (A and B, ×250; C and D, ×600.)
Table 3.
p27kip1 expression in human breast cancers
| Grade | No. of tumors expressing p27kip1
|
% tumors expressing p27kip1
|
||||
|---|---|---|---|---|---|---|
| + | − | Total | + | − | Total | |
| I | 14 | 1 | 15 | 92 | 8 | 100 |
| II | 29 | 7 | 36 | 80 | 20 | 100 |
| III | 10 | 23 | 33 | 30 | 70 | 100 |
p27kip1 expression in human breast tumors ranging from grade I to III. The number of tumors positive and negative for nuclear p27kip1 staining are shown under + or −, respectively. The number of tumors examined for each grade is shown under total. The total number of tumors examined is regarded as 100%, and the percentage of nuclear p27kip1 staining positive and negative tumors is shown as percentage under + or −, respectively for each graded group of tumors.
To see whether the inverse correlation between p27kip1 expression and the degree of malignancy also exists in other types of human tumors, 80 human colon cancer cases were investigated for their p27kip1 expression. A similar expression pattern was seen; this is summarized in Table 4. Nuclear expression of p27kip1 was seen in 82% of Dukes A (9/11), 78% of Dukes B (18/23), 42% of Dukes C (12/29), and 28% of Dukes D (5/17) human colorectal tumors. The statistical study demonstrated that there was a highly significant association of p27kip1 expression and the extend of spread of colorectal cancer (Dukes) (χ2 14.9, P ≪ 0.002). Examples of p27kip1 expression in Dukes A and D colorectal tumors are shown in Fig. 5 C and D.
Table 4.
p27kip1 expression in human colorectal cancers
| Dukes | No. of p27kip1 expressing tumors
|
% of p27kip1 expressing tumors
|
||||
|---|---|---|---|---|---|---|
| + | − | Total | + | − | Total | |
| A | 9 | 2 | 11 | 82 | 18 | 100 |
| B | 18 | 5 | 23 | 78 | 22 | 100 |
| C | 12 | 17 | 29 | 42 | 58 | 100 |
| D | 5 | 12 | 17 | 28 | 72 | 100 |
p27kip1 expression in human colorectal tumors with different stages of malignancy, from Dukes A–D. As in Table 3, the number of tumors positive and negative for nuclear p27kip1 staining are shown under + or −, respectively. The number of tumors examined for each Dukes stage is shown under total. The total number of tumors examined is regarded as 100%, and the percentage of nuclear p27kip1 staining positive and negative tumors is shown as percentage under + or −, respectively for each group of tumors (Dukes A–D).
By studying the expression of p27kip1 in human breast and colorectal tumors with different grades and stages of malignancy, we demonstrated that there is an inverse correlation between the expression of nuclear p27kip1 and the degree of malignancy. The highest p27kip1 expression was detected in the tumors with lowest malignancy—i.e., 92% and 82% of nuclear p27kip1 positive cases in grade I breast tumor and Dukes A colorectal tumors, respectively. Absence of p27kip1 expression was seen mainly in the population of highly malignant breast (70% of grade III) and colorectal (72% of Dukes D) tumors. The specificity of the antibody staining was also controlled using depleted antibody (data not shown). Although the size of the study for each tumor is moderate, the same conclusions were obtained from our analyses carried out in four independent research laboratories and evaluated by four independent pathologists using two different monoclonal anti-p27kip1 antibodies, SX18F7 and SX53G8. The inverse correlation between the expression of p27kip1 and the degree of malignancy seen in human breast and colorectal tumors suggests that p27kip1 expression might be used as a prognostic marker for these human tumors, perhaps allowing therapy to be adjusted more appropriately for individual tumors. Knowing the mechanisms which regulate the high levels expression of p27kip1 in human tumors may in the future provide us with novel strategies to inhibit tumor growth.
Figure 3.
RNase protection assay to detect the p27kip1 mRNA expression in the human breast cancer cell lines as in Fig. 2. The marker is 32P-labeled MspI-digested pBR322 DNA. The 350-bp p27kip1 RNA probe and the β-actin probe from Ambion are labeled as probe. The loading order of protected p27kip1 mRNA from the 12 breast cancer cell lines are the following. Lanes 1–12 are ZR75-1, SKBR5, SKBR7, MCF-7, MDAMB453, BT20, GI-101, T47D, CAMA, CAL51, ZR75-30, and 734B, respectively. Lane 13 is the protected p27kip1 mRNA from a T lymphoid tumor cell line Jurkat, and the negative control yeast RNA is labeled as indicated. (Lower) The protected β-actin mRNA from the same RNase protection assay is used as a RNA loading control.
Acknowledgments
We thank Drs. J. Massague and G. Peters for p27kip1 plasmid and cyclin D1, cdk4 antibodies, respectively. We also thank Dr. Robert Stein for preparing RNA and Prof. P. Farrell for reading the manuscript. The work was mainly supported by Ludwig Institute for Cancer Research. J.A.R. is supported by York CRC. A.M.M. and P.A.H. are funded by European Union Grant PL931619.
ABBREVIATION
- GST
glutathione S-transferase
References
- 1.Draetta G F. Curr. Opin Cell Biol. 1994;6:842–846. doi: 10.1016/0955-0674(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 2.Elledge S J, Harper J W. Curr Opin Cell Biol. 1994;6:847–852. doi: 10.1016/0955-0674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 3.Polyak K, Lee M, Erdjument-Bromaga H, Koff A, Roberts J M, Tempst P, Massague J. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 4.Polyak K, Kato J, Solomon M J, Sherr C J, Massague J, Roberts J M, Koff A. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 5.Reynisdottir I, Polyak K, Iavarone A, Massague J. Genes Dev. 1995;9:1831–1845. doi: 10.1101/gad.9.15.1831. [DOI] [PubMed] [Google Scholar]
- 6.Nourse J, Firpo E, Flanagan W M, Coats S, Polyak K, Lee M H, Massague J, Crabtree G R, Roberts J M. Nature (London) 1994;372:570–573. doi: 10.1038/372570a0. [DOI] [PubMed] [Google Scholar]
- 7.Kato J, Matsuoka M, Polyak K, Massague M, Sherr C. Cell. 1994;79:487–496. doi: 10.1016/0092-8674(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 8.Firpo E J, Koff A, Solomon M J, Roberts J M. Mol Cell Biol. 1994;14:4889–4901. doi: 10.1128/mcb.14.7.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fero M L, Rivkin M, Tasch M, Porter P, Carcow C E, Firpo E, Polyak K, Tsai L H, Broudy V, Perlmutter R M, Kaushansky K, Roberts J. Cell. 1996;85:733–744. doi: 10.1016/s0092-8674(00)81239-8. [DOI] [PubMed] [Google Scholar]
- 10.Kiyokawa H, Kineman R D, Manova-Todorova K O, Soares V C, Hoffman E S, Ono M, Khanam D, Hayday A C, Frohman L A, Koff A. Cell. 1996;85:721–732. doi: 10.1016/s0092-8674(00)81238-6. [DOI] [PubMed] [Google Scholar]
- 11.Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido M, Horii I, Loh D Y, Nakayama K. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 12.Ferando A A, Balbin M, Pendas A M, Vizoso F, Velasco G, Lopez-Otin C. Hum Genet. 1996;97:91–94. doi: 10.1007/BF00218840. [DOI] [PubMed] [Google Scholar]
- 13.Ponce-Castaneda M V, Lee M H, Latres E, Polyak K, Lacombe L, Montgomery K, Mathew S, Krauter K, Scheinfeld J, Massague J. Cancer Res. 1995;55:1211–1214. [PubMed] [Google Scholar]
- 14.Kawamata N, Morosetti R, Miller C W, Park D, Spirin K S, Nakamaki, Takeuchi S, Hatta Y, Simpson J, Wilcyznski S. Cancer Res. 1995;55:2266–2269. [PubMed] [Google Scholar]
- 15.Morosetti R, Kawamata N, Gombart A F, Miller C W, Hatta Y, Hirama T. Blood. 1995;86:1924–1930. [PubMed] [Google Scholar]
- 16.Pietenopol J A, Bohlander S K, Sato Y, Papadopoulos N, Liu B, Friedman C. Cancer Res. 1995;55:1206–1210. [PubMed] [Google Scholar]
- 17.Hengst L, Reed S I. Science. 1996;271:1861–1864. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- 18.Pagano M, Tam S W, Theodoras A M, Beer-Romero P, Sal G D, Chau V, Yew P R, Draetta G F, Rolfe M. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 19.Fredersdorf S, Milne A W, Hall P A, Lu X. Am J Pathol. 1996;148:825–835. [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke C, Titley J, Davies S, O’Hare M J. Epithelial Cell Biol. 1994;3:38–46. [PubMed] [Google Scholar]
- 21.Lukas J, Pagano M, Staskova Z, Draetta G, Bartek J. Oncogene. 1994;9:707–718. [PubMed] [Google Scholar]
- 22.Harlow E, Lane D. Antibodies: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. [Google Scholar]
- 23.Gillett C, Smith P, Gregory W, Richards M, Millis R, Peters G, Barnes D. Int J Cancer. 1996;69:92–99. doi: 10.1002/(SICI)1097-0215(19960422)69:2<92::AID-IJC4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 24.Gillett C, Fantl V, Smith R, Fisher C, Bartek J, Dickson C, Barnes D, Peters G. Cancer Res. 1994;54:1812–1817. [PubMed] [Google Scholar]
- 25.Deng C, Zhang P, Harper J W, Elledge S J, Leder P. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 26.Depoortere F, Dumont J E, Roger P P. J Cell Sci. 1996;108:1759–1764. doi: 10.1242/jcs.109.7.1759. [DOI] [PubMed] [Google Scholar]
- 27.Han E K-H, Begemann M, Sagambato A, Soh J-W, Doki Y, Xing W-Q, Liu W, Weinstein B. Cell Growth Differ. 1996;7:699–710. [PubMed] [Google Scholar]