Abstract
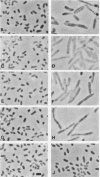
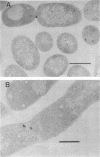
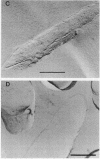
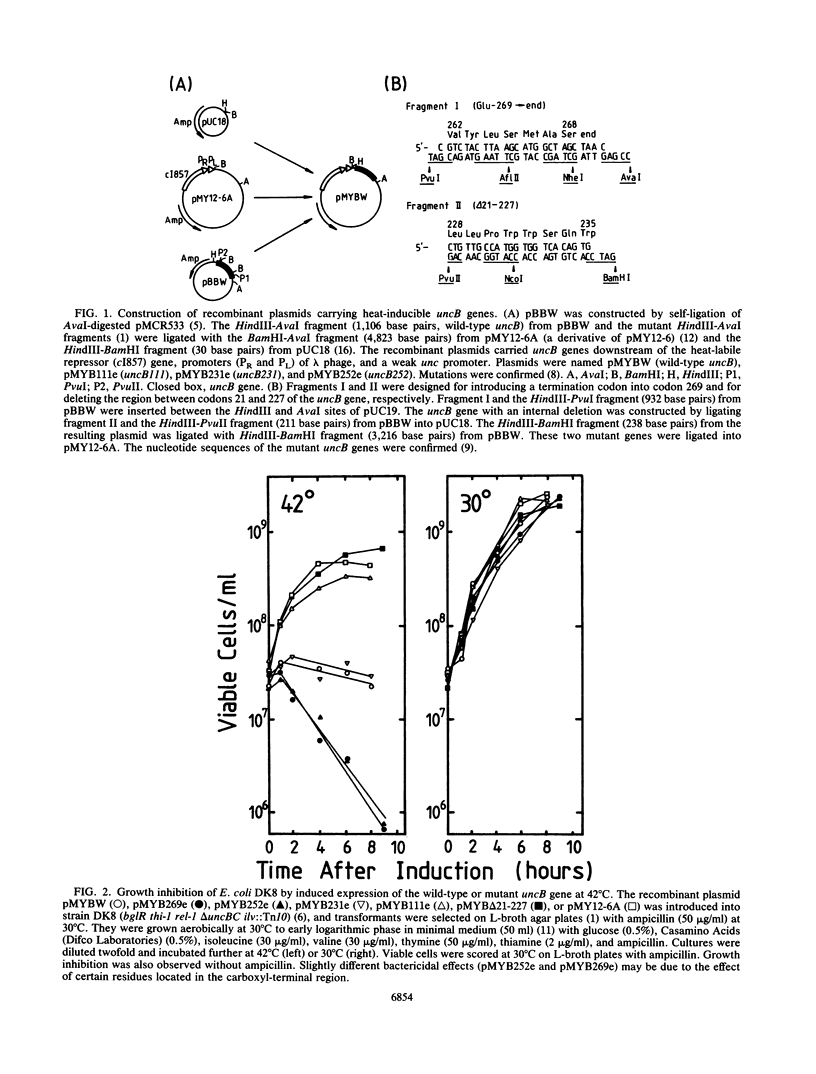
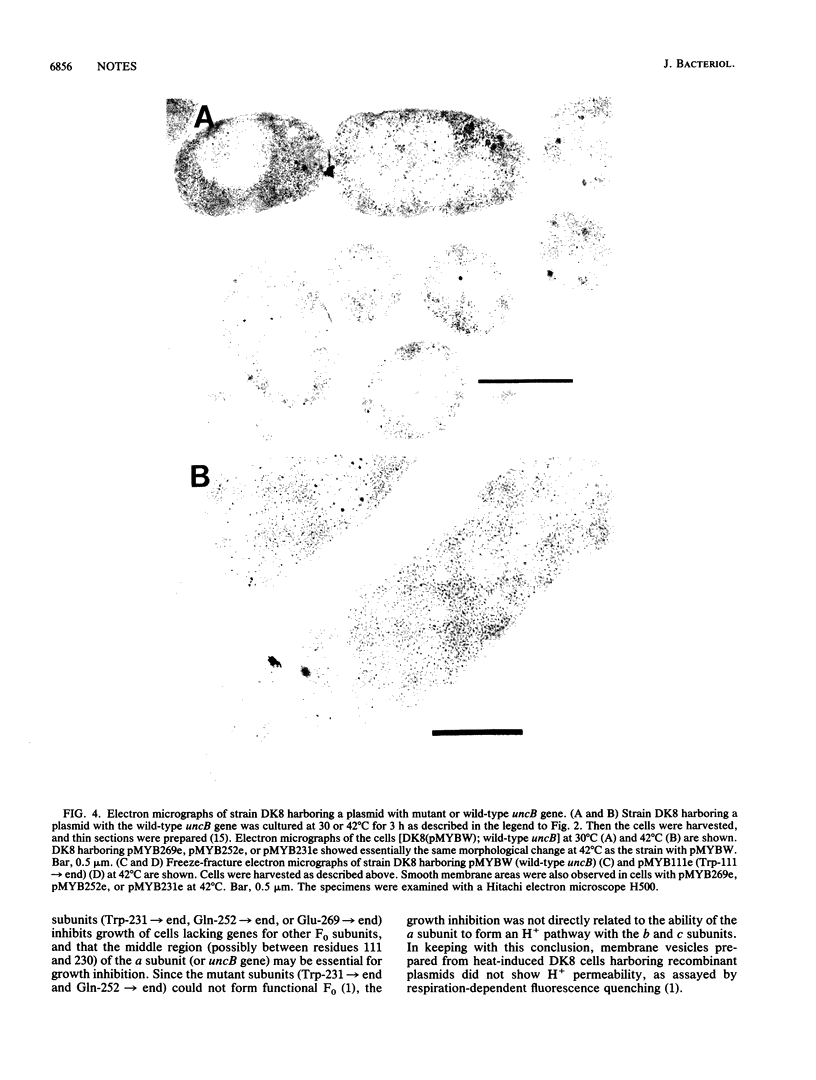
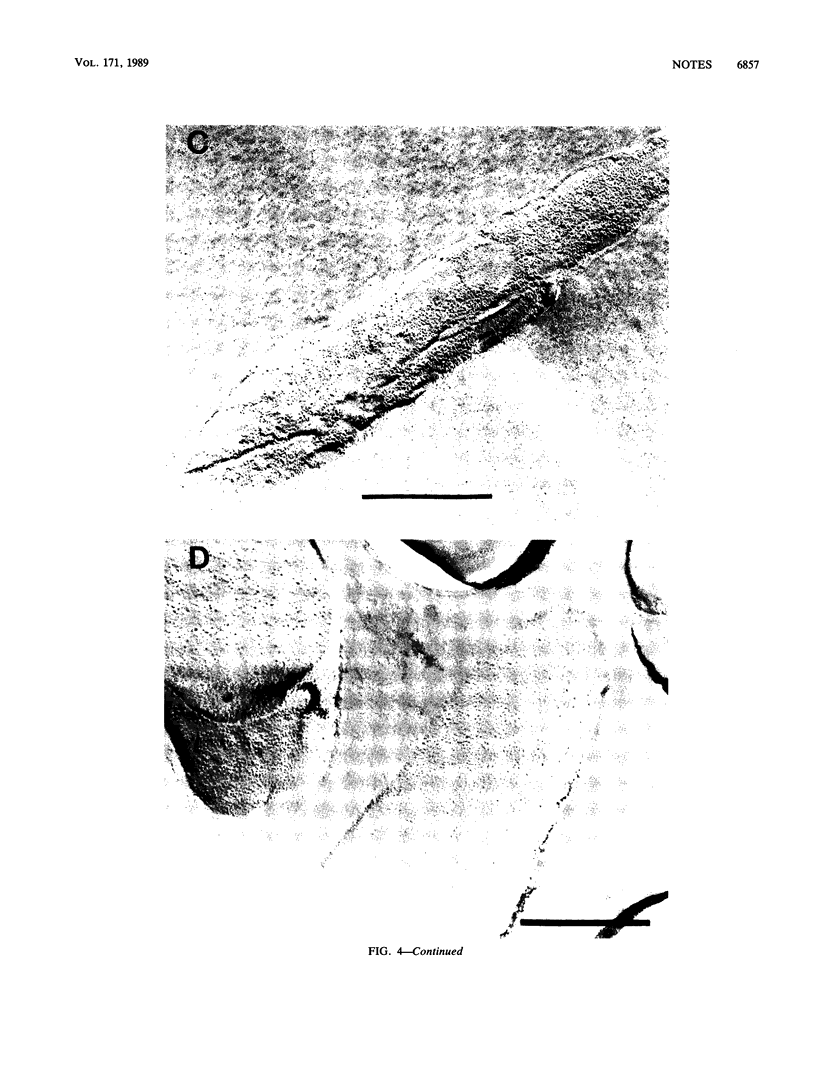
Genes (uncB) for wild-type and mutant a subunits of Escherichia coli H+-ATPase (F0F1) were cloned into recombinant plasmids. The subunits were expressed under the control of a weak promoter of the unc operon at 30 degrees C and strong promoters of lambda phage at 42 degrees C. At 30 degrees C, the wild type and a truncated (Glu-269----end) a subunit complemented the defect of the a subunit mutant KF24A (Trp-111----end), whereas the other mutant subunits (Trp-111----end, Trp-231----end, Gln-252----end, and a subunit with a deletion of residues 21 to 227) did not. Three mutant subunits (Trp-231----end, Gln-252----end, and Glu-269----end) and the wild-type a subunit caused growth inhibition associated with cell elongation, an uneven distribution of membrane proteins, and an altered septum structure when they were expressed at 42 degrees C. These phenomena were not observed with the other mutant subunits, suggesting that overproduction of the middle region (between residues 111 and 230) of the a subunit causes growth inhibition.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eya S., Noumi T., Maeda M., Futai M. Intrinsic membrane sector (Fo) of H+-ATPase (FoF1) from Escherichia coli. Mutations in the alpha subunit give Fo with impaired proton translocation and F1 binding. J Biol Chem. 1988 Jul 25;263(21):10056–10062. [PubMed] [Google Scholar]
- Friedl P., Hoppe J., Gunsalus R. P., Michelsen O., von Meyenburg K., Schairer H. U. Membrane integration and function of the three F0 subunits of the ATP synthase of Escherichia coli K12. EMBO J. 1983;2(1):99–103. doi: 10.1002/j.1460-2075.1983.tb01388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futai M., Kanazawa H. Structure and function of proton-translocating adenosine triphosphatase (F0F1): biochemical and molecular biological approaches. Microbiol Rev. 1983 Sep;47(3):285–312. doi: 10.1128/mr.47.3.285-312.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa H., Kiyasu T., Noumi T., Futai M. Overproduction of subunit a of the F0 component of proton-translocating ATPase inhibits growth of Escherichia coli cells. J Bacteriol. 1984 Apr;158(1):300–306. doi: 10.1128/jb.158.1.300-306.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa H., Tamura F., Mabuchi K., Miki T., Futai M. Organization of unc gene cluster of Escherichia coli coding for proton-translocating ATPase of oxidative phosphorylation. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7005–7009. doi: 10.1073/pnas.77.12.7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D. J., Brusilow W. S., Simoni R. D. In vivo evidence for the role of the epsilon subunit as an inhibitor of the proton-translocating ATPase of Escherichia coli. J Bacteriol. 1984 Dec;160(3):1055–1060. doi: 10.1128/jb.160.3.1055-1060.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noumi T., Futai M., Kanazawa H. Replacement of serine 373 by phenylalanine in the alpha subunit of Escherichia coli F1-ATPase results in loss of steady-state catalysis by the enzyme. J Biol Chem. 1984 Aug 25;259(16):10076–10079. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schneider E., Altendorf K. All three subunits are required for the reconstitution of an active proton channel (F0) of Escherichia coli ATP synthase (F1F0). EMBO J. 1985 Feb;4(2):515–518. doi: 10.1002/j.1460-2075.1985.tb03658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurimoto T., Hase T., Matsubara H., Matsubara K. Bacteriophage lambda initiators: preparation from a strain that overproduces the O and P proteins. Mol Gen Genet. 1982;187(1):79–86. doi: 10.1007/BF00384387. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Gay N. J. The unc operon. Nucleotide sequence, regulation and structure of ATP-synthase. Biochim Biophys Acta. 1984 Sep 6;768(2):164–200. doi: 10.1016/0304-4173(84)90003-x. [DOI] [PubMed] [Google Scholar]
- Weigand R. A., Holt S. C., Shively J. M., Decker G. L., Greenawalt J. W. Ultrastructural properties of the extra membranes of Escherichia coli O111a as revealed by freeze-fracturing and negative-staining techniques. J Bacteriol. 1973 Jan;113(1):433–444. doi: 10.1128/jb.113.1.433-444.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- von Meyenburg K., Jørgensen B. B., Michelsen O., Sørensen L., McCarthy J. E. Proton conduction by subunit a of the membrane-bound ATP synthase of Escherichia coli revealed after induced overproduction. EMBO J. 1985 Sep;4(9):2357–2363. doi: 10.1002/j.1460-2075.1985.tb03939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]