Abstract
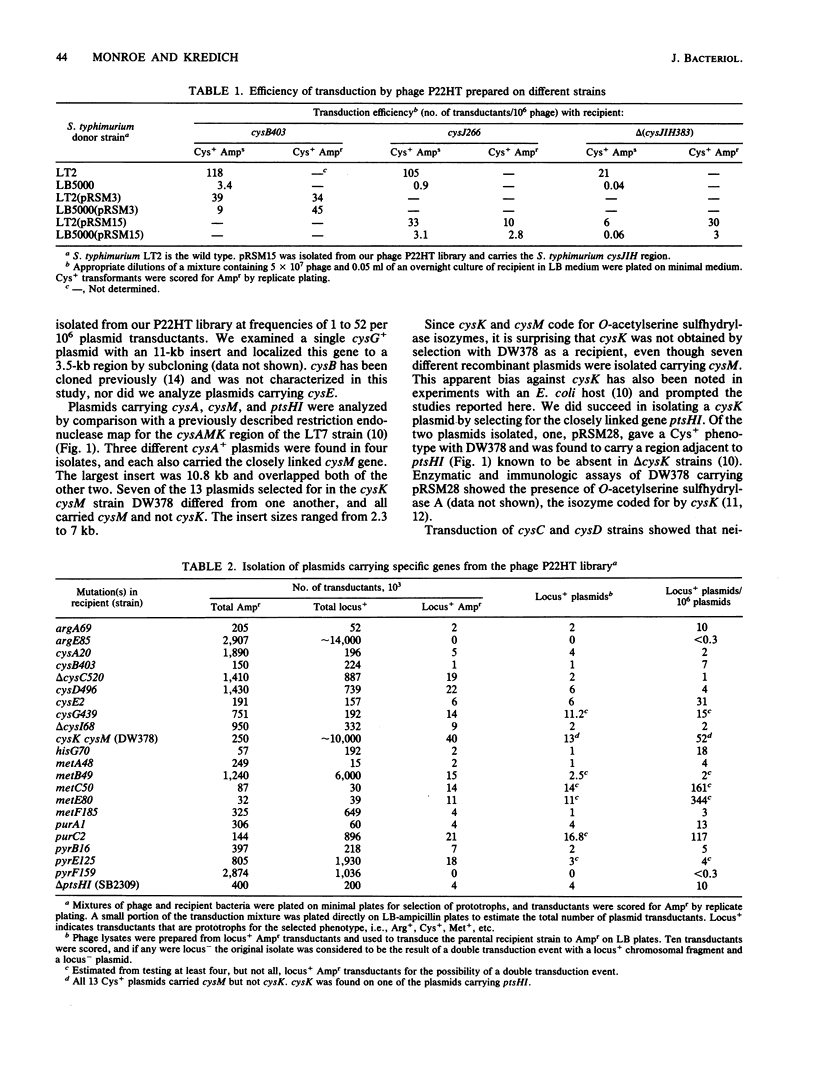
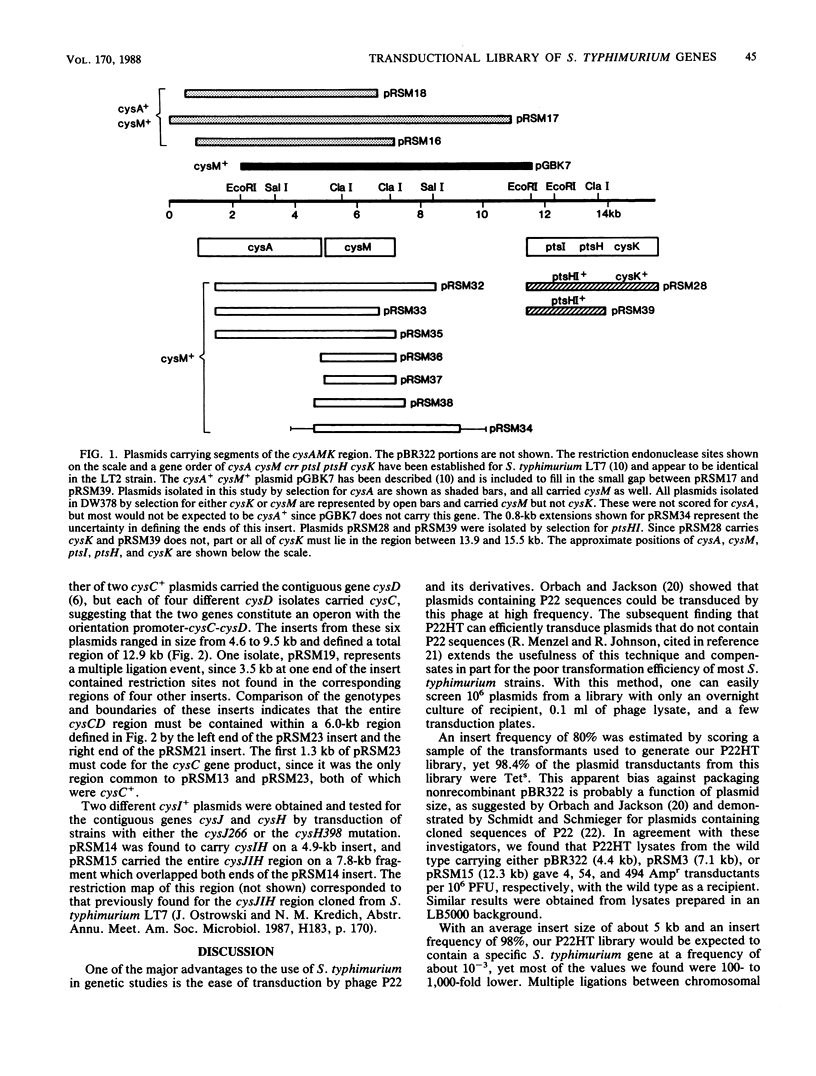
We have prepared a library of Salmonella typhimurium genomic fragments cloned in pBR322 and packaged in P22HT capsids. Plasmids carrying 24 of 26 specific genes searched for were isolated by transduction at frequencies of 1 to 344 per 10(6) plasmid transductants. All 11 known genes of the cysteine regulon were isolated from this library, including cysK, which we had previously been unable to clone in a recombinant plasmid with an Escherichia coli host. This library provides a simple and rapid method for isolating most S. typhimurium genes by using S. typhimurium itself as a host and should be particularly useful for cloning genes that might be deleterious to E. coli.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenthal R. M., Gregory S. A., Cooperider J. S. Cloning of a restriction-modification system from Proteus vulgaris and its use in analyzing a methylase-sensitive phenotype in Escherichia coli. J Bacteriol. 1985 Nov;164(2):501–509. doi: 10.1128/jb.164.2.501-509.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boronat A., Britton P., Jones-Mortimer M. C., Kornberg H. L., Lee L. G., Murfitt D., Parra F. Location on the Escherichia coli genome of a gene specifying O-acetylserine (thiol)-lyase. J Gen Microbiol. 1984 Mar;130(3):673–685. doi: 10.1099/00221287-130-3-673. [DOI] [PubMed] [Google Scholar]
- Bullas L. R., Ryu J. I. Salmonella typhimurium LT2 strains which are r- m+ for all three chromosomally located systems of DNA restriction and modification. J Bacteriol. 1983 Oct;156(1):471–474. doi: 10.1128/jb.156.1.471-474.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelala C. A., Margolin P. Evidence that HT mutant strains of bacteriophage P22 retain an altered form of substrate specificity in the formation of transducing particles in Salmonella typhimurium. Genet Res. 1976 Apr;27(2):315–322. doi: 10.1017/s0016672300016505. [DOI] [PubMed] [Google Scholar]
- Cordaro J. C., Roseman S. Deletion mapping of the genes coding for HPr and enzyme I of the phosphoenolpyruvate: sugar phosphotransferase system in Salmonella typhimurium. J Bacteriol. 1972 Oct;112(1):17–29. doi: 10.1128/jb.112.1.17-29.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMEREC M., GILLESPIE D. H., MIZOBUCHI K. GENETIC STRUCTURE OF THE CYST REGION OF THE SALMONELLA GENOME. Genetics. 1963 Aug;48:997–1009. doi: 10.1093/genetics/48.8.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKASAWA T., NIKAIDO H. Formation of phage receptors induced by galactose in a galactose-sensitive mutant of Salmonella. Virology. 1960 Jun;11:508–510. doi: 10.1016/0042-6822(60)90093-3. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Vapnek D. A simple method of preparing large amounts of phiX174 RF 1 supercoiled DNA. Biochim Biophys Acta. 1973 Apr 11;299(4):516–520. doi: 10.1016/0005-2787(73)90223-2. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hulanicka M. D., Garrett C., Jagura-Burdzy G., Kredich N. M. Cloning and characterization of the cysAMK region of Salmonella typhimurium. J Bacteriol. 1986 Oct;168(1):322–327. doi: 10.1128/jb.168.1.322-327.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulanicka M. D., Hallquist S. G., Kredich N. M., Mojica-A T. Regulation of O-acetylserine sulfhydrylase B by L-cysteine in Salmonella typhimurium. J Bacteriol. 1979 Oct;140(1):141–146. doi: 10.1128/jb.140.1.141-146.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulanicka M. D., Kredich N. M., Treiman D. M. The structural gene for O-acetylserine sulfhydrylase A in Salmonella typhimurium. Identity with the trzA locus. J Biol Chem. 1974 Feb 10;249(3):867–872. [PubMed] [Google Scholar]
- Jackson E. N., Jackson D. A., Deans R. J. EcoRI analysis of bacteriophage P22 DNA packaging. J Mol Biol. 1978 Jan 25;118(3):365–388. doi: 10.1016/0022-2836(78)90234-6. [DOI] [PubMed] [Google Scholar]
- Jagura-Burdzy G., Kredich N. M. Cloning and physical mapping of the cysB region of Salmonella typhimurium. J Bacteriol. 1983 Aug;155(2):578–585. doi: 10.1128/jb.155.2.578-585.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredich N. M. Regulation of L-cysteine biosynthesis in Salmonella typhimurium. I. Effects of growth of varying sulfur sources and O-acetyl-L-serine on gene expression. J Biol Chem. 1971 Jun 10;246(11):3474–3484. [PubMed] [Google Scholar]
- MacLachlan P. R., Sanderson K. E. Transformation of Salmonella typhimurium with plasmid DNA: differences between rough and smooth strains. J Bacteriol. 1985 Jan;161(1):442–445. doi: 10.1128/jb.161.1.442-445.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbach M. J., Jackson E. N. Transfer of chimeric plasmids among Salmonella typhimurium strains by P22 transduction. J Bacteriol. 1982 Mar;149(3):985–994. doi: 10.1128/jb.149.3.985-994.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, Edition VI. Microbiol Rev. 1983 Sep;47(3):410–453. doi: 10.1128/mr.47.3.410-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C., Schmieger H. Selective transduction of recombinant plasmids with cloned pac sites by Salmonella phage P22. Mol Gen Genet. 1984;196(1):123–128. doi: 10.1007/BF00334103. [DOI] [PubMed] [Google Scholar]
- Schmieger H. Packaging signals for phage P22 on the chromosome of Salmonella typhimurium. Mol Gen Genet. 1982;187(3):516–518. doi: 10.1007/BF00332637. [DOI] [PubMed] [Google Scholar]
- Schmieger H. Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet. 1972;119(1):75–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- ZINDER N. D. Bacterial transduction. J Cell Physiol Suppl. 1955 May;45(Suppl 2):23–49. doi: 10.1002/jcp.1030450504. [DOI] [PubMed] [Google Scholar]


