Abstract
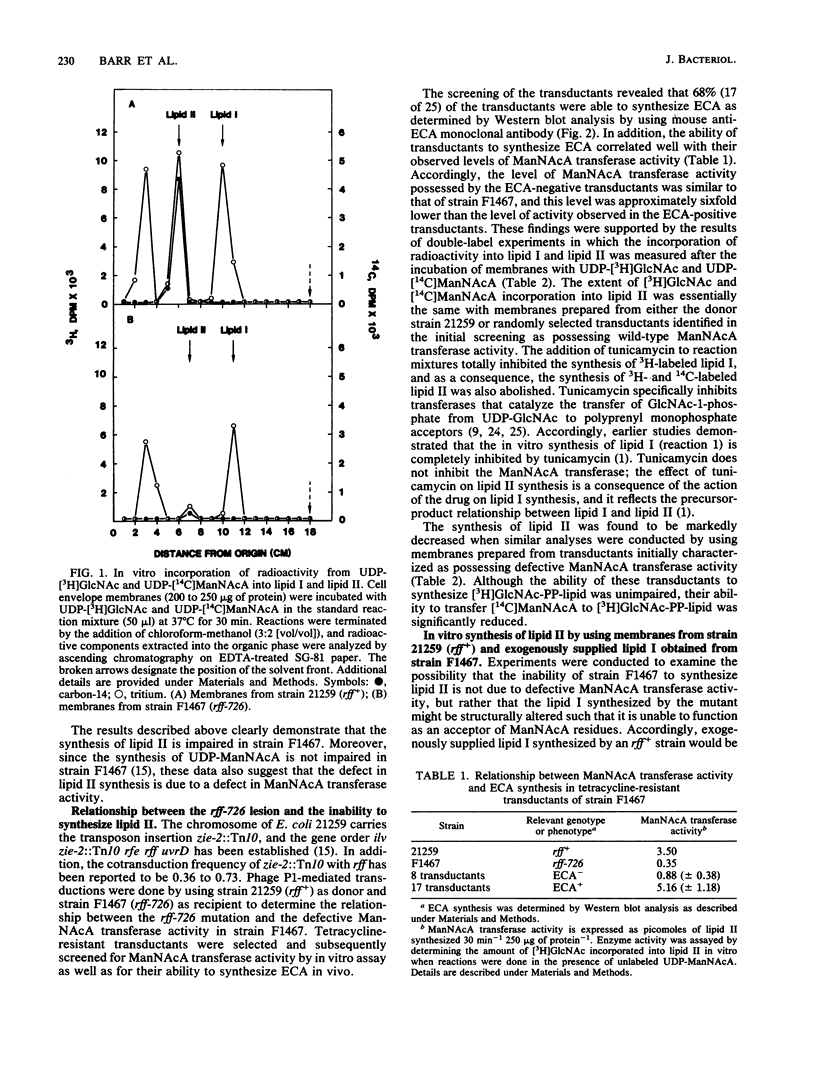
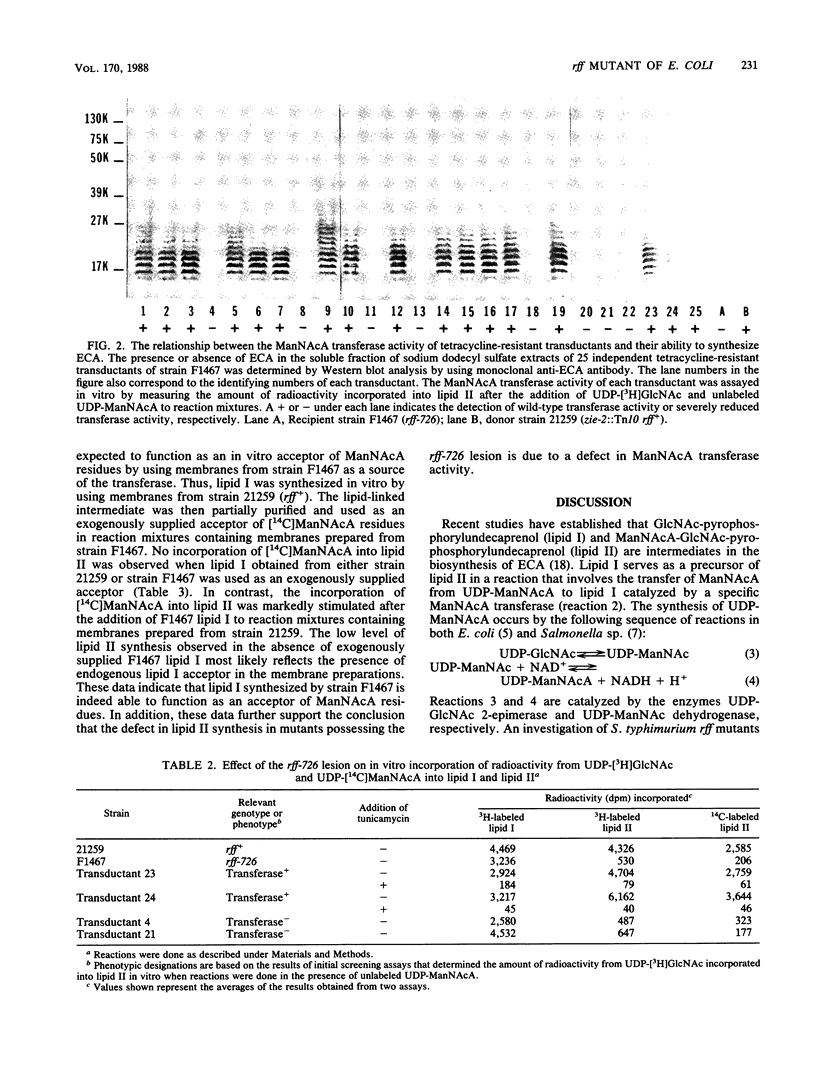
The rff genes of Salmonella typhimurium include structural genes for enzymes involved in the conversion of UDP N-acetyl-D-glucosamine (UDP-GlcNAc) to UDP N-acetyl-D-mannosaminuronic acid (UDP-ManNAcA), the donor of ManNAcA residues in enterobacterial common antigen (ECA) synthesis. An rff mutation (rff-726) of Escherichia coli has been described (U. Meier and H. Mayer, J. Bacteriol. 163:756-762, 1985) that abolished ECA synthesis but which did not affect the synthesis of UDP-ManNAcA or any other components of ECA. The nature of the enzymatic defect resulting from the rff-726 lesion was investigated in the present study. The in vitro synthesis of GlcNAc-pyrophosphorylundecaprenol (lipid I), an early intermediate in ECA synthesis, was demonstrated by using membranes prepared from a mutant of E. coli possessing the rff-726 lesion. However, in vitro synthesis of the next lipid-linked intermediate in the biosynthetic sequence, ManNAcA-GlcNAc-pyrophosphorylundecaprenol (lipid II), was severely impaired. Transduction of wild-type rff genes into the mutant restored the ability to synthesize both lipid II and ECA as determined by in vitro assay and Western blot (immunoblot) analyses done with anti-ECA monoclonal antibody, respectively. Our results are consistent with the conclusion that the rff-726 mutation is located in the structural gene for the transferase that catalyzes the transfer of ManNAcA from UDP-ManNAcA to lipid I.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr K., Rick P. D. Biosynthesis of enterobacterial common antigen in Escherichia coli. In vitro synthesis of lipid-linked intermediates. J Biol Chem. 1987 May 25;262(15):7142–7150. [PubMed] [Google Scholar]
- Basu S., Kuhn H. M., Neszmelyi A., Himmelspach K., Mayer H. Chemical characterization of enterobacterial common antigen isolated from Plesiomonas shigelloides ATCC 14029. Eur J Biochem. 1987 Jan 2;162(1):75–81. doi: 10.1111/j.1432-1033.1987.tb10544.x. [DOI] [PubMed] [Google Scholar]
- Behrens N. H., Tábora E. Dolichol intermediates in the glycosylation of proteins. Methods Enzymol. 1978;50:402–435. doi: 10.1016/0076-6879(78)50047-5. [DOI] [PubMed] [Google Scholar]
- Ichihara N., Ishimoto N., Ito E. Enzymatic formation of uridine diphosphate N-acetyl-D-mannosaminuronic acid. FEBS Lett. 1974 Feb 1;39(1):46–48. doi: 10.1016/0014-5793(74)80013-x. [DOI] [PubMed] [Google Scholar]
- Lew H. C., Mäkelä P. H., Kuhn H. M., Mayer H., Nikaido H. Biosynthesis of enterobacterial common antigen requires dTDPglucose pyrophosphorylase determined by a Salmonella typhimurium rfb gene and a Salmonella montevideo rfe gene. J Bacteriol. 1986 Nov;168(2):715–721. doi: 10.1128/jb.168.2.715-721.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew H. C., Nikaido H., Mäkelä P. H. Biosynthesis of uridine diphosphate N-acetylmannosaminuronic acid in rff mutants of Salmonella tryphimurium. J Bacteriol. 1978 Oct;136(1):227–233. doi: 10.1128/jb.136.1.227-233.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney W. C., Duksin D. Biological activities of the two major components of tunicamycin. J Biol Chem. 1979 Jul 25;254(14):6572–6576. [PubMed] [Google Scholar]
- Mayer H., Schmidt G. Chemistry and biology of the enterobacterial common antigen (ECA). Curr Top Microbiol Immunol. 1979;85:99–153. doi: 10.1007/978-3-642-67322-1_3. [DOI] [PubMed] [Google Scholar]
- Meier U., Mayer H. Genetic location of genes encoding enterobacterial common antigen. J Bacteriol. 1985 Aug;163(2):756–762. doi: 10.1128/jb.163.2.756-762.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä P. H., Jahkola M., Lüderitz O. A new gene cluster rfe concerned with the biosynthesis of Salmonella lipopolysaccharide. J Gen Microbiol. 1970 Jan;60(1):91–106. doi: 10.1099/00221287-60-1-91. [DOI] [PubMed] [Google Scholar]
- Mäkelä P. H., Mayer H. Enterobacterial common antigen. Bacteriol Rev. 1976 Sep;40(3):591–632. doi: 10.1128/br.40.3.591-632.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä P. H., Schmidt G., Mayer H., Nikaido H., Whang H. Y., Neter E. Enterobacterial common antigen in rfb deletion mutants of Salmonella typhimurium. J Bacteriol. 1976 Sep;127(3):1141–1149. doi: 10.1128/jb.127.3.1141-1149.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters H., Jürs M., Jann B., Jann K., Timmis K. N., Bitter-Suermann D. Monoclonal antibodies to enterobacterial common antigen and to Escherichia coli lipopolysaccharide outer core: demonstration of an antigenic determinant shared by enterobacterial common antigen and E. coli K5 capsular polysaccharide. Infect Immun. 1985 Nov;50(2):459–466. doi: 10.1128/iai.50.2.459-466.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTHFIELD L., OSBORN M. J., HORECKER B. L. BIOSYNTHESIS OF BACTERIAL LIPOPOLYSACCHARIDE. II. INCORPORATION OF GLUCOSE AND GALACTOSE CATALYZED BY PARTICULATE AND SOLUBLE ENZYMES IN SALMONELLA. J Biol Chem. 1964 Sep;239:2788–2795. [PubMed] [Google Scholar]
- Rick P. D., Mayer H., Neumeyer B. A., Wolski S., Bitter-Suermann D. Biosynthesis of enterobacterial common antigen. J Bacteriol. 1985 May;162(2):494–503. doi: 10.1128/jb.162.2.494-503.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rick P. D., Osborn M. J. Lipid A mutants of Salmonella typhimurium. Characterization of a conditional lethal mutant in 3-deoxy-D-mannooctulosonate-8-phosphate synthetase. J Biol Chem. 1977 Jul 25;252(14):4895–4903. [PubMed] [Google Scholar]
- Rick P. D., Young D. A. Isolation and characterization of a temperature-sensitive lethal mutant of Salmonella typhimurium that is conditionally defective in 3-deoxy-D-manno-octulosonate-8-phosphate synthesis. J Bacteriol. 1982 May;150(2):447–455. doi: 10.1128/jb.150.2.447-455.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G., Mayer H., Mäkelä P. H. Presence of rfe genes in Escherichia coli: their participation in biosynthesis of O antigen and enterobacterial common antigen. J Bacteriol. 1976 Aug;127(2):755–762. doi: 10.1128/jb.127.2.755-762.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]



