Abstract
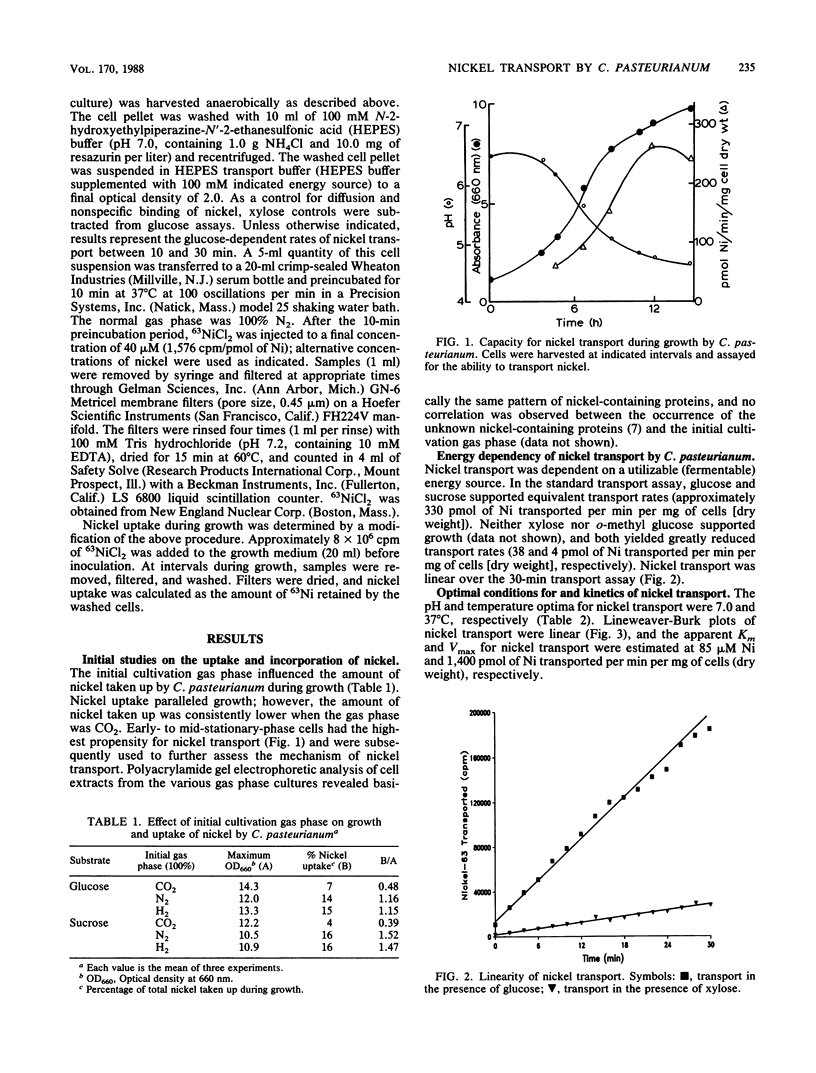
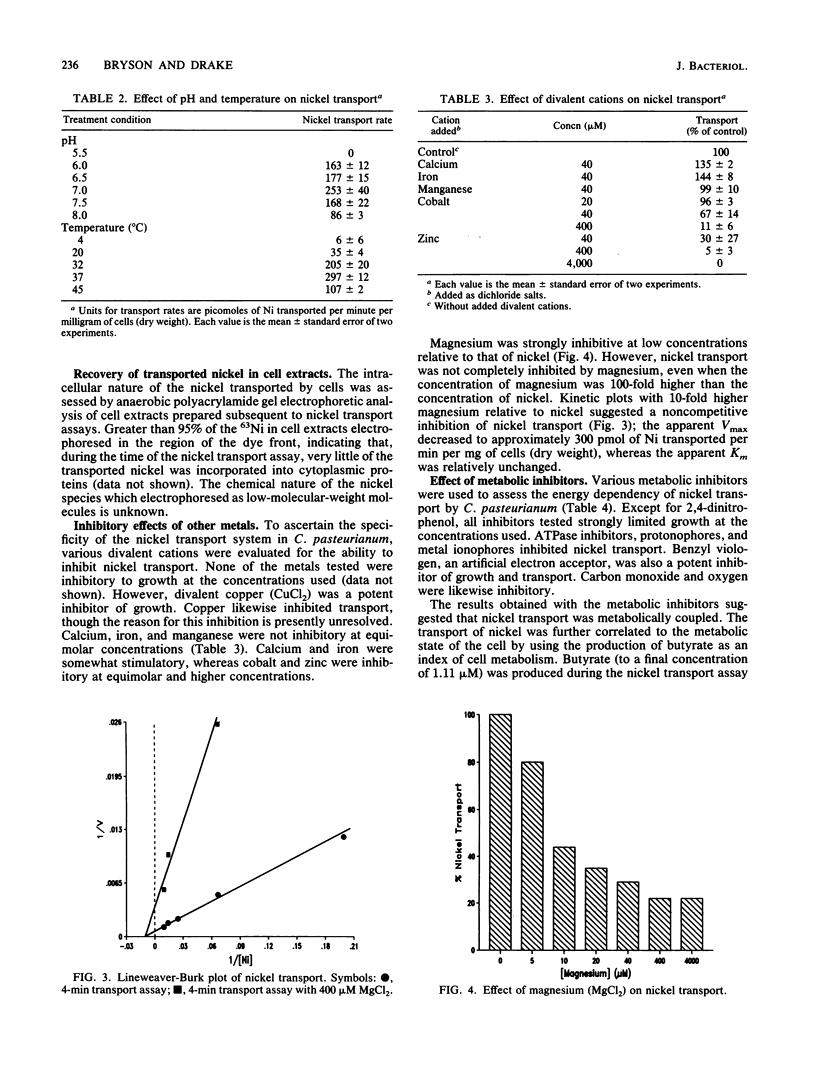
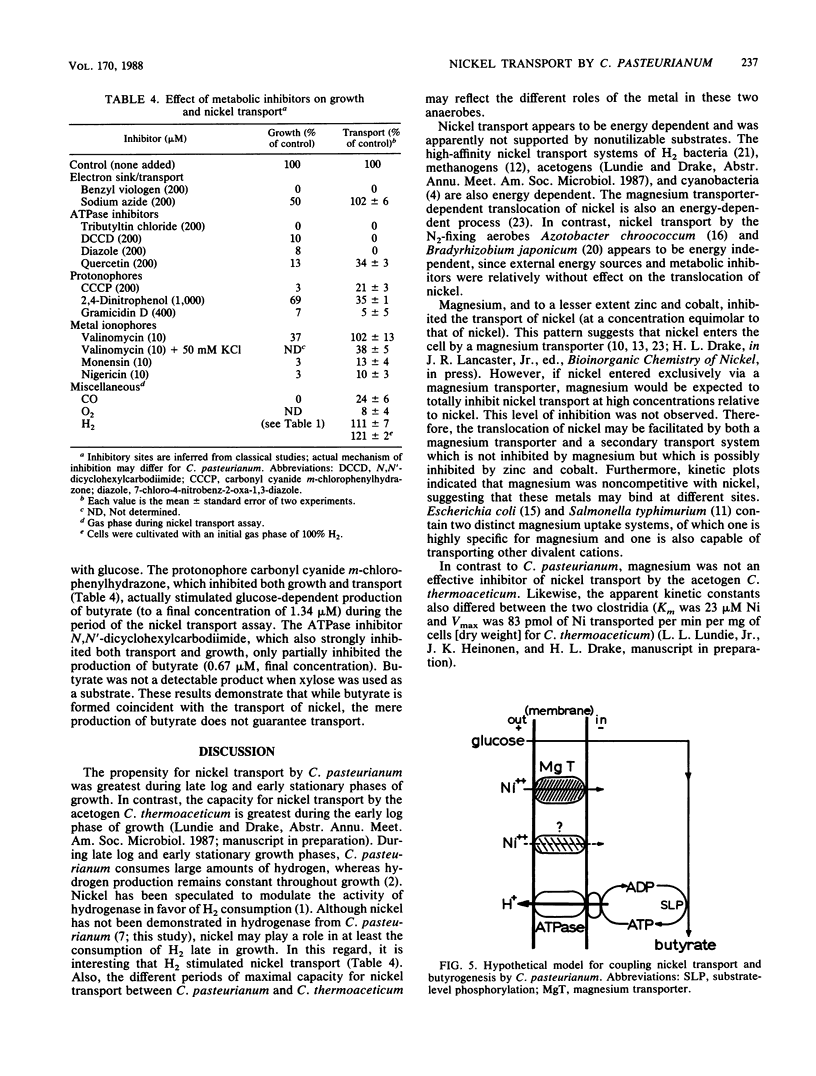
The mechanism of nickel transport by Clostridium pasteurianum was investigated by using 63NiCl2 and a microfiltration transport assay. Nickel transport was energy dependent, requiring either glucose or sucrose; xylose and o-methyl glucose did not support growth, butyrogenesis, or transport. Transport was optimum at pH 7 and 37 degrees C, and early-stationary-phase cells had the highest propensity for nickel transport. The apparent Km and Vmax for nickel transport approximated 85 microM Ni and 1,400 pmol of Ni transported per min per mg (dry weight) of cells, respectively. On the basis of metal specificity, nickel appears to be transported primarily by a magnesium transporter, although an alternative nickel transporter may also be involved. ATPase inhibitors (N,N'-dicyclohexylcarbodiimide, tributyltin chloride, 7-chloro-4-nitrobenz-2-oxa-1,3-diazole, and quercetin), protonophores (carbonyl cyanide m-chlorophenylhydrazone, 2,4-dinitrophenol, and gramicidin D), metal ionophores (valinomycin, monensin, and nigericin), benzyl viologen, carbon monoxide, and oxygen inhibited nickel transport. Nickel transport was coupled indirectly to butyrogenesis and was dependent on the energy state of the cell.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. W., Mortenson L. E. The physical and catalytic properties of hydrogenase II of Clostridium pasteurianum. A comparison with hydrogenase I. J Biol Chem. 1984 Jun 10;259(11):7045–7055. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Campbell P. M., Smith G. D. Transport and accumulation of nickel ions in the cyanobacterium Anabaena cylindrica. Arch Biochem Biophys. 1986 Feb 1;244(2):470–477. doi: 10.1016/0003-9861(86)90615-6. [DOI] [PubMed] [Google Scholar]
- Drake H. L. Demonstration of hydrogenase in extracts of the homoacetate-fermenting bacterium Clostridium thermoaceticum. J Bacteriol. 1982 May;150(2):702–709. doi: 10.1128/jb.150.2.702-709.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake H. L. Occurrence of nickel in carbon monoxide dehydrogenase from Clostridium pasteurianum and Clostridium thermoaceticum. J Bacteriol. 1982 Feb;149(2):561–566. doi: 10.1128/jb.149.2.561-566.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs G., Schnitker U., Thauer R. K. Carbon monoxide oxidation by growing cultures of Clostridium pasteurianum. Eur J Biochem. 1974 Nov 1;49(1):111–115. doi: 10.1111/j.1432-1033.1974.tb03816.x. [DOI] [PubMed] [Google Scholar]
- Hausinger R. P. Nickel utilization by microorganisms. Microbiol Rev. 1987 Mar;51(1):22–42. doi: 10.1128/mr.51.1.22-42.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hmiel S. P., Snavely M. D., Miller C. G., Maguire M. E. Magnesium transport in Salmonella typhimurium: characterization of magnesium influx and cloning of a transport gene. J Bacteriol. 1986 Dec;168(3):1444–1450. doi: 10.1128/jb.168.3.1444-1450.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell K. F., Sprott G. D. Nickel transport in Methanobacterium bryantii. J Bacteriol. 1982 Sep;151(3):1195–1203. doi: 10.1128/jb.151.3.1195-1203.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungdahl L. G. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu Rev Microbiol. 1986;40:415–450. doi: 10.1146/annurev.mi.40.100186.002215. [DOI] [PubMed] [Google Scholar]
- Park M. H., Wong B. B., Lusk J. E. Mutants in three genes affecting transport of magnesium in Escherichia coli: genetics and physiology. J Bacteriol. 1976 Jun;126(3):1096–1103. doi: 10.1128/jb.126.3.1096-1103.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge C. D., Yates M. G. Effect of chelating agents on hydrogenase in Azotobacter chroococcum. Evidence that nickel is required for hydrogenase synthesis. Biochem J. 1982 Apr 15;204(1):339–344. doi: 10.1042/bj2040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebeling V., Thauer R. K., Jungermann K. The internal-alkaline pH gradient, sensitive to uncoupler and ATPase inhibitor, in growing Clostridium pasteurianum. Eur J Biochem. 1975 Jul 1;55(2):445–453. doi: 10.1111/j.1432-1033.1975.tb02181.x. [DOI] [PubMed] [Google Scholar]
- Savage M. D., Drake H. L. Adaptation of the acetogen Clostridium thermoautotrophicum to minimal medium. J Bacteriol. 1986 Jan;165(1):315–318. doi: 10.1128/jb.165.1.315-318.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stults L. W., Mallick S., Maier R. J. Nickel uptake in Bradyrhizobium japonicum. J Bacteriol. 1987 Apr;169(4):1398–1402. doi: 10.1128/jb.169.4.1398-1402.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabillion R., Kaltwasser H. Energieabhängige 63Ni-Aufnahme bei Alcaligenes eutrophus Stamm H1 und H16. Arch Microbiol. 1977 May 13;113(1-2):145–151. doi: 10.1007/BF00428595. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb M. Interrelationships between the utilization of magnesium and the uptake of other bivalent cations by bacteria. Biochim Biophys Acta. 1970 Nov 24;222(2):428–439. doi: 10.1016/0304-4165(70)90133-9. [DOI] [PubMed] [Google Scholar]



