Abstract
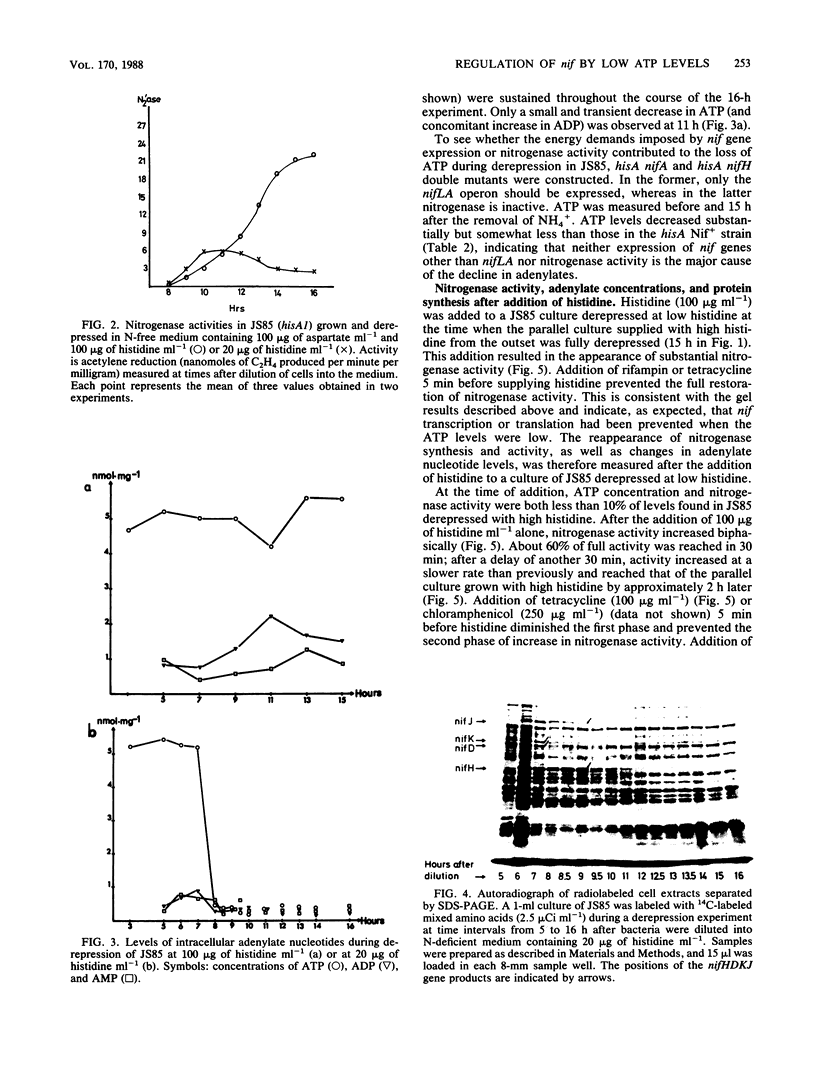
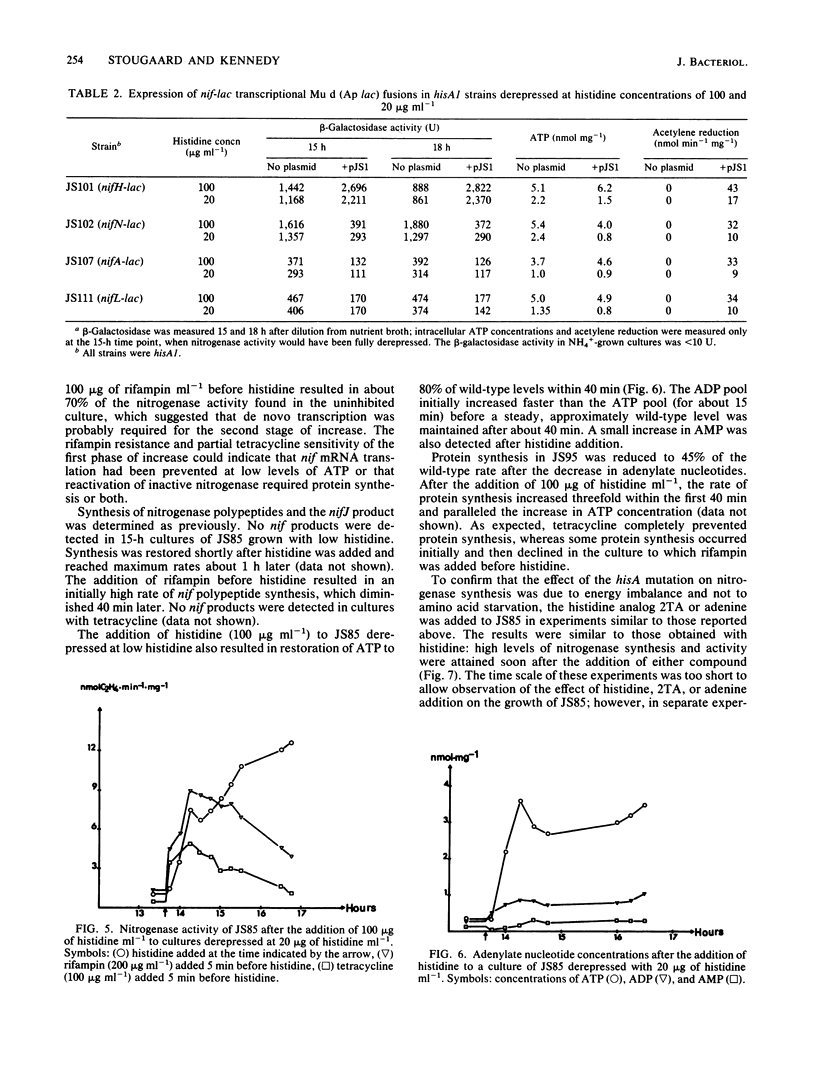
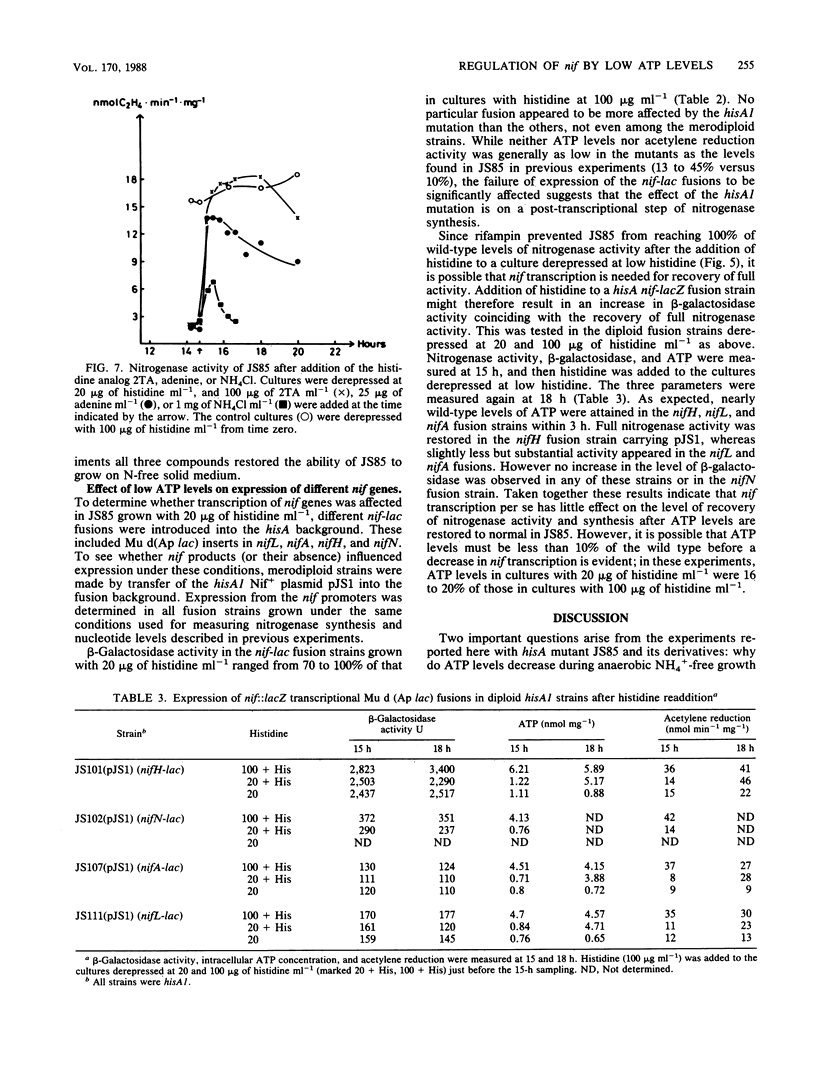
A histidine auxotrophic (hisA) mutant of Klebsiella pneumoniae is phenotypically Nif- when grown with 20 micrograms of histidine ml-1 but Nif+ when supplied with histidine at 100 micrograms ml-1. Reversion to Nif+ at 20 micrograms of histidine ml-1 occurs phenotypically by the addition of 2-thiazolyl-DL-alanine or genetically by mutation in hisG; 2-thiazolyl-DL-alanine inhibits and hisG encodes phosphoribosyl phosphotransferase, the first enzyme of the histidine biosynthetic pathway which consumes ATP. Physiological studies of the hisA mutant JS85 showed that after removal of NH4+ from a culture of the mutant grown with 20 micrograms of histidine ml-1, synthesis of nitrogenase polypeptides occurred at a rate similar to that in the wild type for about 3 h and acetylene reduction activity reached about 10% of the fully derepressed wild-type level. Shortly thereafter the concentration of intracellular adenylates decreased; in particular, ATP fell to about 10% of normal levels. Also, nitrogenase proteins (nifHDK products) and the nifJ gene product stopped being synthesized. These effects were not due to impairment of growth or protein synthesis by histidine starvation. Inhibition of phosphoribosyl phosphotransferase with 2-thiazolyl-DL-alanine restored nitrogenase activity and synthesis, indicating that the effect of the hisA mutation on nif expression was probably a consequence of lowered energy resources that occurred during anaerobic N starvation. The loss of ATP was not associated with nitrogenase synthesis or activity, since hisA nifA and hisA nifH double mutants underwent a loss of ATP in derepressing conditions. Transcription from the nifL, nifN, and nifH promoters was examined in hisA strains with Mu d(Ap lac) fusions in these nif genes. Transcription was not significantly influenced under conditions where adenylates were decreased in concentration. Also nif mRNA apparently accumulated in cultures unable to synthesize nitrogenase, suggesting that translational control of nif gene product synthesis occurs under unfavorable energetic conditions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collins J. J., Roberts G. P., Brill W. J. Posttranscriptional control of Klebsiella pneumoniae nif mRNA stability by the nifL product. J Bacteriol. 1986 Oct;168(1):173–178. doi: 10.1128/jb.168.1.173-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. A. The genetic complexity of nitrogen fixation. The ninth Fleming lecture. J Gen Microbiol. 1984 Nov;130(11):2745–2755. doi: 10.1099/00221287-130-11-2745. [DOI] [PubMed] [Google Scholar]
- Dixon R., Eady R. R., Espin G., Hill S., Iaccarino M., Kahn D., Merrick M. Analysis of regulation of Klebsiella pneumoniae nitrogen fixation (nif) gene cluster with gene fusions. Nature. 1980 Jul 10;286(5769):128–132. doi: 10.1038/286128a0. [DOI] [PubMed] [Google Scholar]
- Galloway R. J., Taylor B. L. Histidine starvation and adenosine 5'-triphosphate depletion in chemotaxis of Salmonella typhimurium. J Bacteriol. 1980 Dec;144(3):1068–1075. doi: 10.1128/jb.144.3.1068-1075.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S., Kennedy C., Kavanagh E., Goldberg R. B., Hanau R. Nitrogen fixation gene (nifL) involved in oxygen regulation of nitrogenase synthesis in K. pneumoniae. Nature. 1981 Apr 2;290(5805):424–426. doi: 10.1038/290424a0. [DOI] [PubMed] [Google Scholar]
- Jensen J. S., Kennedy C. Pleiotropic effect of his gene mutations on nitrogen fixation in Klebsiella pneumoniae. EMBO J. 1982;1(2):197–204. doi: 10.1002/j.1460-2075.1982.tb01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston H. M., Roth J. R. Histidine mutants requiring adenine: selection of mutants with reduced hisG expression in Salmonella typhimurium. Genetics. 1979 May;92(1):1–15. doi: 10.1093/genetics/92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn D., Hawkins M., Eady R. R. Metabolic control of Klebsiella pneumoniae mRNA degradation by the availability of fixed nitrogen. J Gen Microbiol. 1982 Dec;128(12):3011–3018. doi: 10.1099/00221287-128-12-3011. [DOI] [PubMed] [Google Scholar]
- Kaluza K., Hennecke H. Regulation of nitrogenase messenger RNA synthesis and stability in Klebsiella pneumoniae. Arch Microbiol. 1981 Sep;130(1):38–43. doi: 10.1007/BF00527069. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Eady R. R., Kondorosi E., Rekosh D. K. The molybdenum--iron protein of Klebsiella pneumoniae nitrogenase. Evidence for non-identical subunits from peptide 'mapping'. Biochem J. 1976 May 1;155(2):383–389. doi: 10.1042/bj1550383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner D., Phillips S. Relative levels of guanosine 5'-diphosphate 3'-diphosphate (ppGpp) in some N2 fixing bacteria during derepression and repression of nitrogenase. Arch Microbiol. 1981 Jan;128(3):341–342. doi: 10.1007/BF00422542. [DOI] [PubMed] [Google Scholar]
- Lindahl L., Zengel J. M. Ribosomal genes in Escherichia coli. Annu Rev Genet. 1986;20:297–326. doi: 10.1146/annurev.ge.20.120186.001501. [DOI] [PubMed] [Google Scholar]
- Lundin A., Richardsson A., Thore A. Continous monitoring of ATP-converting reactions by purified firefly luciferase. Anal Biochem. 1976 Oct;75(2):611–620. doi: 10.1016/0003-2697(76)90116-0. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA). Proc Natl Acad Sci U S A. 1984 Apr;81(7):1966–1970. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair M. B., Eady R. R. Nitrogenase synthesis in Klebsiella pneumoniae: enhanced nif expression without accumulation of guanosine 5'-diphosphate 3'-diphosphate. J Gen Microbiol. 1984 Dec;130(12):3063–3069. doi: 10.1099/00221287-130-12-3063. [DOI] [PubMed] [Google Scholar]
- Riesenberg D., Erdei S., Kondorosi E., Kari C. Positive involvement of ppGpp in derepression of the nif operon in Klebsiella pneumoniae. Mol Gen Genet. 1982;185(2):198–204. doi: 10.1007/BF00330786. [DOI] [PubMed] [Google Scholar]
- SHEDLOVSKY A. E., MAGASANIK B. A defect in histidine biosynthesis causing an adenine deficiency. J Biol Chem. 1962 Dec;237:3725–3730. [PubMed] [Google Scholar]
- SHEDLOVSKY A. E., MAGASANIK B. The enzymatic basis of an adenine-histidine relationship in Escherichia coli. J Biol Chem. 1962 Dec;237:3731–3736. [PubMed] [Google Scholar]
- Schnaitman C. A., McDonald G. A. Regulation of outer membrane protein synthesis in Escherichia coli K-12: deletion of ompC affects expression of the OmpF protein. J Bacteriol. 1984 Aug;159(2):555–563. doi: 10.1128/jb.159.2.555-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Kleckner N. Translational control of IS10 transposition. Cell. 1983 Sep;34(2):683–691. doi: 10.1016/0092-8674(83)90401-4. [DOI] [PubMed] [Google Scholar]
- Swedes J. S., Sedo R. J., Atkinson D. E. Relation of growth and protein synthesis to the adenylate energy charge in an adenine-requiring mutant of Escherichia coli. J Biol Chem. 1975 Sep 10;250(17):6930–6938. [PubMed] [Google Scholar]
- Tyler B. Regulation of the assimilation of nitrogen compounds. Annu Rev Biochem. 1978;47:1127–1162. doi: 10.1146/annurev.bi.47.070178.005403. [DOI] [PubMed] [Google Scholar]



