Abstract
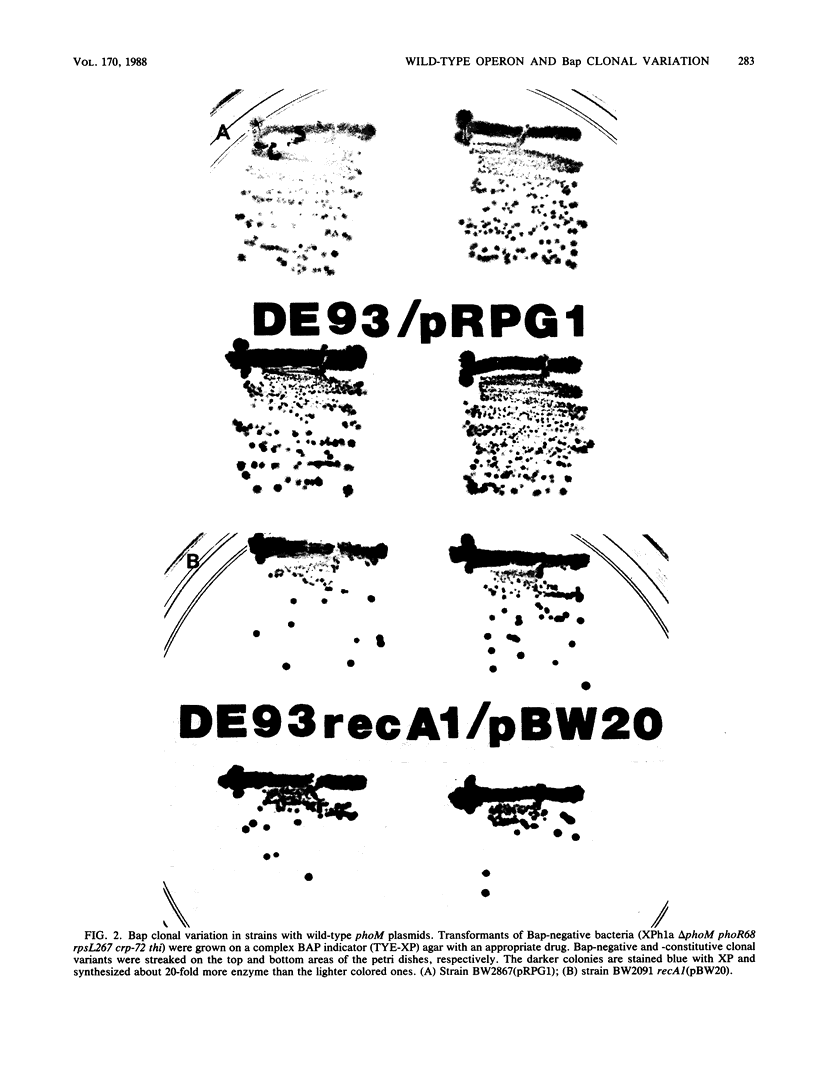
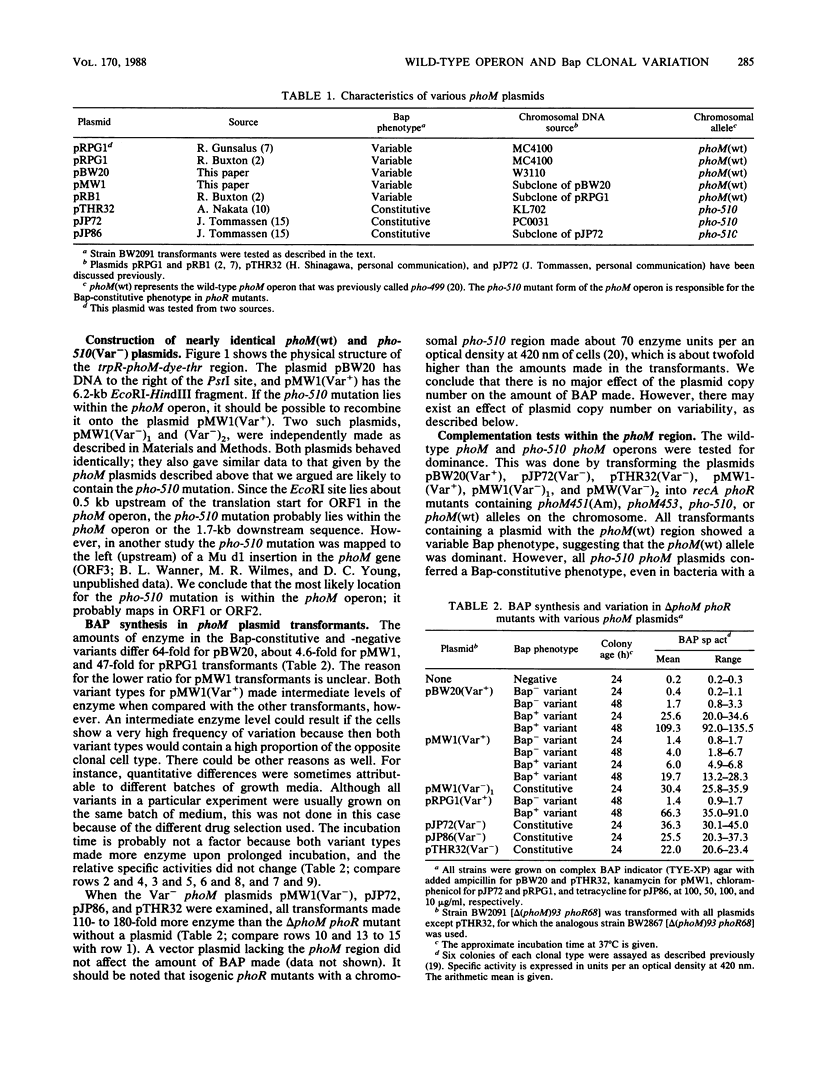
A metastable bacterial alkaline phosphatase (Bap) phenotype is seen in phoR mutants, which alternately express a Bap-constitutive or -negative phenotype. The alteration is affected by mutations in the phoM region near 0 min. By molecular cloning of the wild-type phoM operon onto a multicopy plasmid and recombining onto the plasmid the pho-510 mutation that abolishes variation, the phoM operon, rather than some nearby gene, was shown to control variation. Complementation tests indicated that the wild-type phoM allele is dominant to the pho-510 mutation when both are in single copy, but whichever allele is present in higher copy appears as dominant when multicopy plasmids are examined. The alternating phenotypic variation of BAP synthesis was not seen in phoR+ cells with multicopy wild-type phoM plasmids, thus showing that the variation is associated with phoM-dependent Bap expression. The alternation acted at the level of phoA transcription; it was also recA independent. BAP clonal variation is phenotypically similar to Salmonella phase variation, which is controlled by a DNA rearrangement. No evidence was found for a DNA change near the phoM operon that might be responsible for the variable Bap phenotype.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amemura M., Makino K., Shinagawa H., Nakata A. Nucleotide sequence of the phoM region of Escherichia coli: four open reading frames may constitute an operon. J Bacteriol. 1986 Oct;168(1):294–302. doi: 10.1128/jb.168.1.294-302.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton R. S., Drury L. S. Identification of the dye gene product, mutational loss of which alters envelope protein composition and also affects sex factor F expression in Escherichia coli K-12. Mol Gen Genet. 1984;194(1-2):241–247. doi: 10.1007/BF00383523. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury L. S., Buxton R. S. DNA sequence analysis of the dye gene of Escherichia coli reveals amino acid homology between the dye and OmpR proteins. J Biol Chem. 1985 Apr 10;260(7):4236–4242. [PubMed] [Google Scholar]
- Gunsalus R. P., Yanofsky C. Nucleotide sequence and expression of Escherichia coli trpR, the structural gene for the trp aporepressor. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7117–7121. doi: 10.1073/pnas.77.12.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Zurawski G., Yanofsky C. Structural and functional analysis of cloned deoxyribonucleic acid containing the trpR-thr region of the Escherichia coli chromosome. J Bacteriol. 1979 Oct;140(1):106–113. doi: 10.1128/jb.140.1.106-113.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katinka M., Cossart P., Sibilli L., Saint-Girons I., Chalvignac M. A., Le Bras G., Cohen G. N., Yaniv M. Nucleotide sequence of the thrA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5730–5733. doi: 10.1073/pnas.77.10.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludtke D., Bernstein J., Hamilton C., Torriani A. Identification of the phoM gene product and its regulation in Escherichia coli K-12. J Bacteriol. 1984 Jul;159(1):19–25. doi: 10.1128/jb.159.1.19-25.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino K., Shinagawa H., Nakata A. Cloning and characterization of the alkaline phosphatase positive regulatory gene (phoM) of Escherichia coli. Mol Gen Genet. 1984;195(3):381–390. doi: 10.1007/BF00341438. [DOI] [PubMed] [Google Scholar]
- Marinus M. G., Konrad E. B. Hyper-recombination in dam mutants of Escherichia coli K-12. Mol Gen Genet. 1976 Dec 22;149(3):273–277. doi: 10.1007/BF00268528. [DOI] [PubMed] [Google Scholar]
- Ronson C. W., Nixon B. T., Ausubel F. M. Conserved domains in bacterial regulatory proteins that respond to environmental stimuli. Cell. 1987 Jun 5;49(5):579–581. doi: 10.1016/0092-8674(87)90530-7. [DOI] [PubMed] [Google Scholar]
- Silverman M., Simon M. Phase variation: genetic analysis of switching mutants. Cell. 1980 Apr;19(4):845–854. doi: 10.1016/0092-8674(80)90075-6. [DOI] [PubMed] [Google Scholar]
- Singleton C. K., Roeder W. D., Bogosian G., Somerville R. L., Weith H. L. DNA sequence of the E. coli trpR gene and prediction of the amino acid sequence of Trp repressor. Nucleic Acids Res. 1980 Apr 11;8(7):1551–1560. doi: 10.1093/nar/8.7.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackett L. P., Wanner B. L., Venditti C. P., Walsh C. T. Involvement of the phosphate regulon and the psiD locus in carbon-phosphorus lyase activity of Escherichia coli K-12. J Bacteriol. 1987 Apr;169(4):1753–1756. doi: 10.1128/jb.169.4.1753-1756.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L. Bacterial alkaline phosphatase clonal variation in some Escherichia coli K-12 phoR mutant strains. J Bacteriol. 1986 Dec;168(3):1366–1371. doi: 10.1128/jb.168.3.1366-1371.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., Bernstein J. Determining the phoM map location in Escherichia coli K-12 by using a nearby transposon Tn10 insertion. J Bacteriol. 1982 Apr;150(1):429–432. doi: 10.1128/jb.150.1.429-432.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., Chang B. D. The phoBR operon in Escherichia coli K-12. J Bacteriol. 1987 Dec;169(12):5569–5574. doi: 10.1128/jb.169.12.5569-5574.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L. Control of phoR-dependent bacterial alkaline phosphatase clonal variation by the phoM region. J Bacteriol. 1987 Feb;169(2):900–903. doi: 10.1128/jb.169.2.900-903.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., Latterell P. Mutants affected in alkaline phosphatase, expression: evidence for multiple positive regulators of the phosphate regulon in Escherichia coli. Genetics. 1980 Oct;96(2):353–366. doi: 10.1093/genetics/96.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., McSharry R. Phosphate-controlled gene expression in Escherichia coli K12 using Mudl-directed lacZ fusions. J Mol Biol. 1982 Jul 5;158(3):347–363. doi: 10.1016/0022-2836(82)90202-9. [DOI] [PubMed] [Google Scholar]
- Wanner B. L. Molecular cloning of Mu d(bla lacZ) transcriptional and translational fusions. J Bacteriol. 1987 May;169(5):2026–2030. doi: 10.1128/jb.169.5.2026-2030.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L. Novel regulatory mutants of the phosphate regulon in Escherichia coli K-12. J Mol Biol. 1986 Sep 5;191(1):39–58. doi: 10.1016/0022-2836(86)90421-3. [DOI] [PubMed] [Google Scholar]
- Wanner B. L., Wieder S., McSharry R. Use of bacteriophage transposon Mu d1 to determine the orientation for three proC-linked phosphate-starvation-inducible (psi) genes in Escherichia coli K-12. J Bacteriol. 1981 Apr;146(1):93–101. doi: 10.1128/jb.146.1.93-101.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G. J., Twigg A. J., Sherratt D. J. ColE1 plasmid mobility and relaxation complex. Nature. 1978 Jul 20;274(5668):259–261. doi: 10.1038/274259a0. [DOI] [PubMed] [Google Scholar]
- Weiss D. L., Johnson D. I., Weith H. L., Somerville R. L. Structural analysis of the ileR locus of Escherichia coli K12. J Biol Chem. 1986 Jul 25;261(21):9966–9971. [PubMed] [Google Scholar]




