Abstract
Fas ligand (FasL) plays a pivotal role in lymphocyte cytotoxicity and the maintenance of immunological homeostasis. Since FasL has been implicated in the existence of immunologically privileged body sites by inducing apoptosis of activated T lymphocytes, we investigated the expression of FasL in human colon cancers. We found that two out of seven primary tumors and all four hepatic metastatic tumors of surgically obtained colonic adenocarcinoma expressed FasL mRNA and protein, detected by reverse transcription-coupled PCR and by immunohistochemical staining, respectively. Expression of FasL was not detected in normal colonic epithelial cells. FasL mRNA was also expressed in some human colonic adenocarcinoma cell lines including SW480, SW1116, and LS180 cells. Cell-surface-associated FasL was detected in these human colon cancer cells by fluorescence immunocytochemical staining. In addition, the expressed FasL was demonstrated to be functional, since coculture experiments using FasL-expressing SW480 cells resulted in apoptosis of Jurkat T leukemia cells that are sensitive to Fas-mediated apoptosis, and this process was specifically inhibited by the neutralizing anti-human FasL antibody. Thus, our findings and other data suggest an alternative mechanism that enables tumors to evade immune destruction by inducing apoptosis in activated T lymphocytes. Furthermore, constitutive expression of FasL in hepatic metastatic tumors suggests that FasL may also be important in their colonization in the liver through induction of apoptosis in the surrounding Fas-expressing hepatocytes.
Keywords: tumor escape, immune surveillance, colonization
The Fas ligand (FasL) and its receptor (Fas, CD95) are a set of regulatory components in the immune system (1–4). Activation of the Fas by FasL results in apoptosis of many cell types (5, 6), and this process has been shown to play a critical role in the maintenance of immunological homeostasis and peripheral tolerance by deletion of activated T lymphocytes (7–10). In addition, it has been found that cells in immunologically privileged sites, such as Sertoli’s cells of the testis and parenchymal cells of the anterior chamber of the eye, express FasL. Any activated T cell bearing Fas that enters such a site would encounter cells expressing FasL and receive a death signal, thereby preventing an immune response (11, 12).
It has recently been demonstrated that FasL is expressed in human melanoma (13), hepatocellular carcinoma (14), and a colon cancer cell line (15). These studies suggested that the expression of FasL may play an important role in establishing immunologically privileged environments that allow tumors to escape the host’s immune surveillance. We therefore investigated the expression of FasL in primary colon cancers, liver metastases, and colon cancer cell lines.
We found that FasL was strongly expressed in hepatic metastatic tumors of colonic adenocarcinomas. Since this expressed FasL was functional, it may be important for the elimination of activated T lymphocytes that attempt to attack tumor cells and it may support the belief that FasL plays a pivotal role in the escape of tumor cells from destruction by the host’s immune system (13–17). In addition, the constitutive expression of FasL in hepatic metastatic tumors suggests that it may also play a critical role in the liver colonization of colon cancer cells.
MATERIALS AND METHODS
Cell Lines.
Human colonic adenocarcinoma cell lines LS180, HT-29, Caco-2, SW480, SW403, SW1116, SK-CO-1, COLO320DM, and COLO320HSR and the Jurkat human T leukemia cell line were obtained from American Type Culture Collection. KM12C cells were kindly provided by Isaiah J. Fidler (University of Texas, M.D. Anderson Cancer Center, Houston) (18). Jurkat cells, COLO320DM, and COLO320HSR were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum with penicillin (100 units/ml) and streptomycin (100 μg/ml); all other colon cancer cell lines were maintained in DMEM.
To establish tumor xenografts of human colonic adenocarcinomas, 4- to 6-week-old BALB/c nude mice (Taconic Farms) were injected subcutaneously in the left shoulder with 1 × 106 SW480 cells or KM12C cells in 0.1 ml of sterile 20 mM phosphate-buffered saline (PBS), pH 7.2. When they reached a size of approximately 10 mm in diameter, these tumors were snap-frozen in liquid nitrogen, embedded in OCT compound (Miles Scientific), and stored at −80°C for histologic study.
Human Colonic Adenocarcinoma Tissues.
Primary and hepatic metastatic tumors of colon adenocarcinoma, as well as adjacent normal colonic mucosa counterparts, were obtained fresh at the time of surgery, immediately frozen in liquid nitrogen, and stored at −80°C. Cryostat sections were cut from these snap-frozen tissues, stained with hematoxylin/eosin, and examined histologically to identify and separate tumor and nonneoplastic areas for RNA extraction and immunohistological study.
Detection of FasL Transcript.
The expression of FasL mRNA was determined by reverse transcription (RT) of total RNA followed by PCR analysis (RT-PCR) (19). Approximately 107 cells of human colonic adenocarcinoma cells or 50–100 mg of colon adenocarcinoma tissue from primary and hepatic metastatic tumors was homogenized with 1 ml of Ultraspec RNA reagent (Biotecx Laboratories, Houston) by using a Mini-Beadbeater (Biospec Products, Bartlesville, OK), and total RNA was isolated according to the manufacturer’s protocol. cDNAs were synthesized by extension of (dT)12–18 primers with 200 units of SuperScript II reverse transcriptase (Life Technologies) in a mixture containing 2 μg of total RNA digested by RNase-free DNase I (2 units/μg of RNA) (Promega) for 30 min at 37°C. PCR of the cDNA was performed in a final volume of 50 μl containing all four dNTPs (each at 200 μM), 2.5 mM MgCl2, 2.5 units of AmpliTaq Gold (Perkin–Elmer), and each primer at 0.4 μM. The amplification cycles were 94°C for 30 s, 55°C for 1 min, and 72°C for 1.5 min.
The PCR products were separated by electrophoresis on a 2% agarose gel after 35 cycles (231-bp human FasL fragment) or on a 1.5% agarose gel after 30 cycles (838-bp human β-actin fragment) and visualized by ethidium bromide staining. Amplification of human β-actin served as a control for sample loading and integrity. Primers used for amplification were human FasL sense primer corresponding to nucleotides 483–503 (5′-CTGGGGATGTTTCAGCTCTTC-3′), and antisense primer complementary to nucleotides 713–693 (5′-CTTCACTCCAGAAAGCAGGAC-3′), franking introns 1, 2, and 3 (20, 21), and β-actin sense primer corresponding to nucleotides 578–609 (5′-ATCTGGCACCACACCTTCTACAATGAGCTGCG-3′), and antisense primer complementary to nucleotides 1415–1384 (5′-CGTCATACTCCTGCTTGCTGATCCACATCTGC-3′). Total RNA was obtained from human peripheral T lymphocytes stimulated with ionomycin and phorbol 12-myristate 13-acetate for 24 hr as described (22) and served as a positive control for detection of FasL mRNA.
Detection of FasL by Immunofluorescence Cell Staining.
Human colon adenocarcinoma cells were grown for 48 hr on a sterile circular cover glass (25 mm diameter) placed in the bottom of a six-well culture plate. These live tumor cells were incubated for 1 hr at 4°C with rabbit polyclonal IgG antibody against the carboxyl terminus (anti-FasL C-20) or against the amino terminus (anti-FasL N-20) of human FasL (Santa Cruz Biotechnology) at 1.0 μg/ml. The cells were then incubated with fluorescein isothiocyanate-conjugated goat anti-rabbit IgG secondary antibody (Sigma) at 2.0 μg/ml for 30 min at 4°C, and visualized with a fluorescence microscope after fixation with 2% paraformaldehyde. Similar experiments were performed with permeabilized cells fixed with 2% paraformaldehyde for 1 hr at 4°C and treated with 0.1% saponin in PBS for 15 min at room temperature. Staining with isotype-matched purified immunoglobulin (rabbit IgG; Sigma) that was isolated from pooled normal rabbit serum was performed as a negative control, and all primary antibodies were dissolved in PBS containing 1.5% normal goat serum.
Immunohistochemical Staining.
Cryostat sections of surgically excised human colon cancers or tumor xenografts of cancer cell lines grown in nude mice were cut from snap-frozen tissues embedded in OCT compound (Miles), air-dried onto glass slides at room temperature, and fixed in 100% acetone at −20°C for 5 min. These tissue sections were incubated with anti-FasL N-20 rabbit IgG antibody (0.05 μg/ml) overnight at 4°C. Bound primary antibodies were visualized by the avidin-biotin complex immunoperoxidase method, using the Vectastain ABC Elite peroxidase kit (Vector Laboratories) and 3,3′-diaminobenzidine as chromogen, and counterstained with methyl green.
Apoptosis Assay.
The FasL-expressing SW480 colon cancer cells were subconfluently seeded in 6- or 96-well tissue culture plates (Falcon) and allowed to grow to confluence. The cells were then washed once with sterile PBS and fixed with 2% paraformaldehyde at 4°C for 1 hr. After the cells were washed two times with serum-free RPMI 1640 medium, 2 ml or 200 μl of Jurkat cell suspension (5 × 105 cells per ml of complete RPMI 1640 medium) was added to each 6- or 96-well, respectively.
After 72 hr of coculture, floating Jurkat cells were collected from the six-well tissue culture plates after gentle rocking of the culture plate. After centrifugation, the cell pellets were resuspended in 1.0 ml of hypotonic fluorochrome solution (propidium iodide at 50 μg/ml in 0.1% sodium citrate and 0.1% Triton X-100) and incubated at 4°C for 4 hr in the dark (23). For detection of apoptosis, 20,000 events were measured per sample, using a FACScan flow cytometer (Becton Dickinson). The propidium iodide was excited with 488 nm of argon laser light and was collected as red fluorescence by using a 560-nm dichroic mirror and a 600-nm band pass filter. Percentage of apoptotic cells was determined by evaluating hypodiploid nuclei. The FasL-negative KM12C cells were confluently grown in six-well tissue culture plates, fixed with 2% paraformaldehyde, and used as negative control effector cells for FasL-expressing SW480.
To quantitatively assess the proportion of dead Jurkat cells, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was also performed (24). Briefly, MTT stock solution (2.5 mg/ml in H2O) was added to 96-well culture plates (10 μl per well) at the end of coculture and incubated for 4 hr at 37°C. After removing culture supernatant by centrifugation, a blue formazan product formed in the live cells by oxidation was solubilized by acid isopropanol (0.04 M HCl in isopropanol) and the absorbance of each well was measured with a microculture plate reader (model 7520 microplate reader, Cambridge Technology, Cambridge, MA) at 590 nm.
To block FasL-induced apoptosis, Jurkat cells were cocultured with fixed SW480 cells in the presence of hamster anti-human FasL IgG neutralizing mAb (clone 4H9; MBL International, Watertown, MA) at 1 μg/ml (25) or isotype-matched irrelevant hamster mAb (anti-trinitrophenol IgG mAb, PharMingen). As a control for Fas-mediated apoptosis, Jurkat cells were cultured in the presence of mouse anti-human Fas IgM mAb (clone CH11, MBL International) at 100 ng/ml (26), or irrelevant mouse IgM mAb derived from myeloma cells (Sigma). All antibodies were treated with immobilized endotoxin affinity ligand (END-X endotoxin neutralization resin, Associates of Cape Cod) for overnight at 4°C to remove any possible endotoxin contamination.
RESULTS
FasL mRNA Expression in Human Colonic Adenocarcinoma Cell Lines and Tumor Tissues.
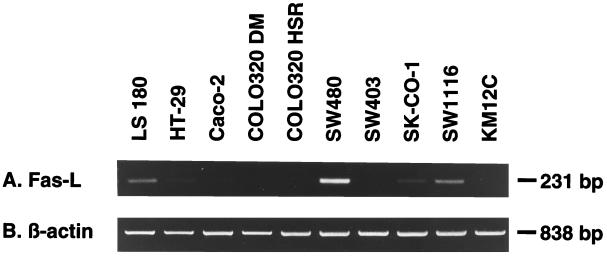
To investigate the expression of FasL mRNA in human colonic adenocarcinoma cell lines and tumor tissues, total RNA was extracted from subconfluent cell cultures or snap-frozen primary tumors and hepatic metastases and analyzed by RT-PCR. As shown in Fig. 1, a 231-bp RT-PCR fragment of FasL transcript was detected by ethidium bromide in LS 180, SW480, and SW1116 human colonic adenocarcinoma cell lines and very weakly detected in HT-29 and SK-CO-1 cells. SW480 cells expressed FasL transcript more strongly than other colon cancer cell lines. FasL transcript was not detected in Caco-2, COLO320 MD, COLO320 HSR, SW403, and KM12C cells.
Figure 1.
Detection of FasL mRNA in human colonic adenocarcinoma cell lines by RT-PCR. A 231-bp human FasL-specific sequence (A) and an 838-bp β-actin sequence (B) were amplified from total RNA isolated from human colon cancer cell lines, separated by agarose gel electrophoresis, and visualized by ethidium bromide staining.
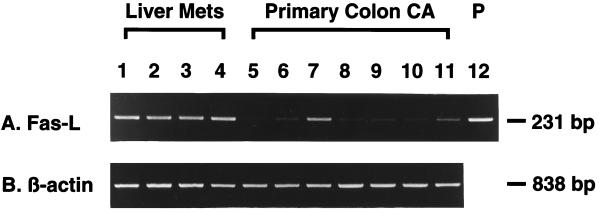
There was strong expression of FasL mRNA in all (4 of 4) hepatic metastatic tumors (Fig. 2, lanes 1–4). In contrast, FasL transcript was detected only in two of seven primary colon cancers (Fig. 2, lanes 7 and 11), and its intensity was less than in the hepatic metastatic tumors (Fig. 2).
Figure 2.
Detection of FasL mRNA in primary and hepatic metastatic human colonic adenocarcinoma tissues by RT-PCR. Agarose gel electrophoresis and ethidium bromide staining of 231-bp FasL RT-PCR products (A) and 838-bp β-actin RT-PCR products (B). Liver Mets, hepatic metastatic tumor tissues of human colonic adenocarcinoma; Primary Colon CA, primary tumor tissues of human colonic adenocarcinoma; P, human FasL cDNA synthesized from total RNA of human peripheral blood T lymphocytes stimulated with ionomycin and phorbol 12-myristate 13-acetate (positive control) (22).
Cell Surface Expression of FasL in Colon Cancer Cell Lines.
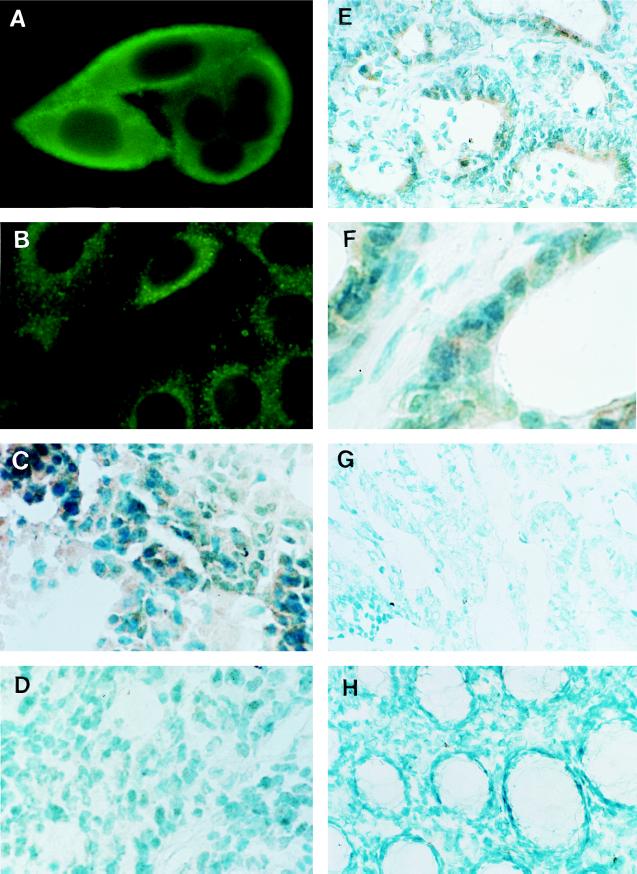
Immunofluorescence cell staining for FasL expressed in human colon cancer cell lines were performed with rabbit polyclonal antibodies against the carboxyl terminus (anti-FasL C-20) and amino terminus (anti-FasL N-20) of FasL. To determine the orientation of FasL protein expressed on the cell surface of human colon cancer cell lines, nonpermeabilized live SW480 cells were stained by these antibodies. FasL was detected only by anti-FasL C-20 antibody (Fig. 3A) but not by anti-FasL N-20 antibody or control rabbit IgG (data not shown). Staining was particularly strong in the outer linings of these nonpermeabilized cells, indicating cell surface localization of FasL. In contrast, when SW480 cells were permeabilized by saponin, their FasL was detected by staining with both anti-FasL C-20 and N-20 antibodies and the staining showed a diffuse cytoplasmic pattern (Fig. 3B). These results were consistent with the previous observation that FasL is a type II membrane protein (amino-terminal is cytoplasmic) (2, 4, 5, 21). Other human cancer cell lines that expressed FasL mRNA (Fig. 1) demonstrated similar staining patterns, but FasL protein was not detected in FasL mRNA-negative KM12C cells by either anti-FasL C-20 or N-20 antibodies (data not shown).
Figure 3.
Expression of FasL protein in human colonic adenocarcinoma. (A) Immunofluorescence staining of live SW480 human colonic adenocarcinoma cells with anti-FasL C-20 polyclonal antibody (×1,000). (B) Immunofluorescence staining of permeabilized SW480 cells with anti-FasL N-20 polyclonal antibody (×1,000). (C and D) Immunoperoxidase staining of cryostat sections of SW480 (C) and KM12C (D) xenograft tumors with anti-FasL N-20 polyclonal antibody (×400). (E and F) Immunoperoxidase staining of hepatic metastatic lesion of human colonic adenocarcinoma tissue (case 4 in Fig. 2) with anti-FasL N-20 polyclonal antibody (×200). F is a higher magnification (×400) of E. Note that especially as seen in F, every tumor cell strongly expressed FasL protein. (G and H) Immunoperoxidase staining of primary tumor of human colonic adenocarcinoma tissue (G) and normal human colon mucosa (H) with anti-FasL N-20 polyclonal antibody (×200).
To exclude the possibility that FasL expression in human colonic adenocarcinoma cell lines was a consequence of in vitro culture conditions, FasL expression was examined in the xenograft of SW480 and KM12C cells grown subcutaneously in nude mice by immunoperoxidase staining using anti-FasL N-20 antibody. FasL was detected in solid tumors of SW480 cells (Fig. 3C) but not in tumors of KM12C (Fig. 3D). No staining was observed with a nonspecific control antibody (data not shown).
Expression of FasL in Colon Cancer Tissues.
Expression of FasL protein in liver metastases and primary colon cancer tissues was examined by immunoperoxidase staining using anti-FasL N-20 antibody. Figs. 3 E and F show typical staining patterns of hepatic metastatic tumors for FasL. The staining of FasL was limited to the colon cancer cells and demonstrated a diffuse cellular staining pattern (Fig. 3 E and F). All (4 of 4) liver metastases expressed FasL protein. In contrast, the majority (5 of 7) of the primary tumors expressed minimal, if any, FasL protein (Fig. 3G). There was very little or no staining in normal colon mucosa (Fig. 3H).
Induction of Lymphocyte Apoptosis.
To determine whether or not FasL expressed in colonic adenocarcinoma is functional, coculture was performed between FasL-expressing SW480 cells and Jurkat cells of Fas-expressing T cell leukemia cells (10, 14, 15, 27). As demonstrated in Fig. 4A, untreated Jurkat cells showed a typical diploid DNA peak. Jurkat cells cocultured with FasL-positive SW480 cells showed a significant reduction of the number of nuclei with diploid DNA content with a marked increase in the hypodiploid DNA peak, indicating apoptosis of Jurkat cells (14, 23). This shift in the nuclear content of DNA was not observed when Jurkat cells were cocultured with FasL-negative KM12C cells.
Figure 4.
Apoptosis of Jurkat cells induced by SW480 cells. (A) FACScan analysis of propidium iodide staining of nucleus of Jurkat cells cocultured with SW480 cells (Jurkat + SW480) or with KM12C cells (Jurkat + KM12C). After coculture (72 hr), Jurkat cells were isolated and stained with propidium iodine. The DNA content of Jurkat cells was examined by flow cytometry. (B) MTT assay of Jurkat cells alone or cocultivated with SW480 cells in the presence of irrelevant mouse IgM mAb (irrelevant IgM), mouse anti-human Fas IgM mAb (anti-Fas IgM), hamster anti-trinitrophenol mAb (irrelevant IgG), or hamster anti-human FasL IgG neutralizing mAb (anti-FasL IgG).
The cytotoxicity of SW480 cells to Jurkat cells was also quantified by MTT assay. Viability of Jurkat cells decreased notably when they were cocultured with SW480 cells (Fig. 4B). This cytotoxicity was mediated by FasL, because it was inhibited by a neutralizing anti-human FasL mAb, but not by an irrelevant control mAb (Fig. 4B).
DISCUSSION
FasL is a member of the tumor necrosis factor family (2, 4, 5, 21) and mediates apoptosis of many cell types, including both transformed and nontransformed cells (1, 5, 6, 28). FasL has been shown to play a pivotal role in lymphocyte cytotoxicity (29–31) and is also important in mediating autocrine suicide in activated T lymphocytes (3, 7–10, 32). Since tumor progression and the metastatic process may require an evasion of host immune surveillance, we investigated whether colon cancers express FasL. We found that FasL mRNA was expressed in several human colon cancer cell lines (3 of 10) and primary tumors (2 of 7). Surprisingly all (4 of 4) liver metastases expressed FasL mRNA strongly. Protein expression of FasL was confirmed by immunoperoxidase staining. This observation contrasts with the lack of expression of Fas in liver metastases (data not shown). It was also demonstrated that the expressed FasL is functional; FasL-expressing SW480 human colonic adenocarcinoma cells induced apoptosis of Jurkat T cells, and this process was specifically inhibited by treatment with a neutralizing anti-human FasL antibody. These findings are consistent with those reported by O’Connell et al. (15) that SW620 human colon cancer cells express functional FasL.
It has been shown previously that the number of tumor-infiltrating lymphocytes in primary colorectal carcinomas decreases in parallel with tumor progression and metastasis (33). This observation supports the hypothesis that local anti-tumor immunity conferred by the infiltrating lymphocytes may play an important role in controlling progression and metastasis (34, 35). Furthermore, it has recently been proposed that FasL expressed on tumor cells may be responsible for the elimination of tumor-infiltrating lymphocytes in vivo (13), promoting progression and metastasis through the evasion of the host immune response. In this regard, the production of FasL by murine T cell lymphoma cells transfected with FasL gene has been show to induce a CD8+ T cell-mediated protective immunity against subsequent challenge with FasL-negative parental tumor cells in vivo (36); it will be interesting to determine whether or not FasL-expressing T cell lymphoma cells escape from this protective immunity by induction of apoptosis in CD8+ T cells.
We propose that FasL expressed on the surface of colonic adenocarcinoma cells may have an additional role in malignancy; namely, FasL may be important in the colonization of colon cancer cells in the liver, a major target of metastasis (37). The metastatic cascade is a complex series of processes including angiogenesis, intravasation of tumor cells, transport by the circulation, adhesive interaction with endothelial cells, extravasation, and colonization of the target organ (38–41). Only a small subpopulation of cells from heterogeneous primary tumors appears able to form metastatic colonies (42, 43). The survival and proliferation of these metastatic cells depend on various biological properties, such as resistance to host defense mechanisms, regulation of adhesion molecules, and enzymes that degrade basement membranes (39, 40, 43, 44). In addition, liver colonization competence is a critical determinant in colon cancer metastasis (41). FasL expression on colon cancer cells may enhance liver colonization competence through induction of apoptosis in the Fas-expressing hepatocytes (45–47). This concept was indirectly supported by our finding that FasL was expressed strongly in all the hepatic metastatic tumors tested (4 of 4), wherease less than 30% of the primary colon cancer cells expressed FasL. Thus, FasL expression on tumor cells may be important in enhancing liver colonization through the induction of apoptosis of the hepatocytes at metastatic foci.
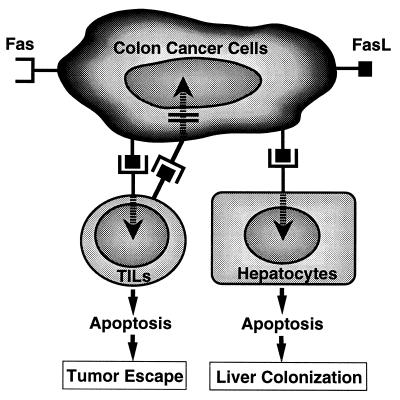
Fig. 5 summarizes a proposed working hypothesis on the FasL-mediated colon cancer–host interactions, as derived from our present data and from the previous literature. FasL-expressing colon cancer cells may induce apoptosis of tumor-infiltrating lymphocytes as well as of hepatocytes but not of cancer cells themselves, which are generally resistant to Fas-mediated apoptosis (15, 48). Thus, FasL in colon cancer cells may play a pivotal role in escaping the host’s immune system and promoting liver colonization of colonic adenocarcinoma. Further evaluation of these possible roles of FasL and the regulation of FasL expression in malignant cells should be critically important for the development of new strategies for controlling the growth of malignant cells that escape host immune surveillance and spread to the liver.
Figure 5.
Hypothetical models of FasL-expressing human colonic adenocarcinomas in tumor escape and liver colonization.
Acknowledgments
This work was supported by Grants CA57584 and NIDDK43351 from the National Institutes of Health and an Educational Grant from Toray Industries, Inc. (Japan).
ABBREVIATIONS
- FasL
Fas ligand
- RT
reverse transcription
- MTT
3(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
References
- 1.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S. Cell. 1991;66:233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 2.Suda T, Takahashi T, Golstein P, Nagata S. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 3.Lynch D H, Ramsdell F, Alderson M R. Immunol Today. 1995;16:569–574. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 4.Nagata S, Suda S. Immunol Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 5.Nagata S, Golstein P. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 6.Cleveland J L, Ihle J N. Cell. 1995;81:479–482. doi: 10.1016/0092-8674(95)90068-3. [DOI] [PubMed] [Google Scholar]
- 7.Singer G G, Abbas A K. Immunity. 1994;1:365–371. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 8.Alderson M R, Tough T W, Davis-Smith T, Braddy S, Falk B, Schooley K A, Goodwin R G, Smith C A, Ramsdell F, Lynch D H. J Exp Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunner T, Mogil R J, LaFace D, Yoo N J, Mahboubi A, Echeverri F, Martin S J, Force W R, Lynch D H, Ware C F, Green D R. Nature (London) 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 10.Dhein J, Walczak H, Baumler C, Debatin K M, Krammer P H. Nature (London) 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 11.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke R C. Nature (London) 1995;377:630–632. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 12.Griffith T S, Brunner T, Fletcher S M, Green D R, Ferguson T A. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 13.Hahne M, Rimoldi D, Schröter M, Romero P, Schreier M, French L E, Schneider P, Bornand T, Fontana A, Lienard D, Cerottini J C, Tschopp J. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 14.Strand S, Hofmann W J, Hug H, Müller M, Otto G, Strand D, Mariani S M, Stremmel W, Krammer P H, Galle P R. Nat Med. 1996;2:1361–1366. doi: 10.1038/nm1296-1361. [DOI] [PubMed] [Google Scholar]
- 15.O’Connell J, O’Sullivan G C, Collins J K, Shanahan F. J Exp Med. 1996;184:1075–1082. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Streilein J W. Science. 1995;270:1158–1159. doi: 10.1126/science.270.5239.1158. [DOI] [PubMed] [Google Scholar]
- 17.Williams N. Science. 1996;274:1302. doi: 10.1126/science.274.5291.1302. [DOI] [PubMed] [Google Scholar]
- 18.Morikawa K, Walker S M, Jessup J M, Fidler I J. Cancer Res. 1988;48:1943–1948. [PubMed] [Google Scholar]
- 19.Chelly J, Kaplan J C, Maire P, Gautron S, Kahn A. Nature (London) 1988;333:858–860. doi: 10.1038/333858a0. [DOI] [PubMed] [Google Scholar]
- 20.Mita E, Hayashi N, Iio S, Takehara T, Hijioka T, Kasahara A, Fusamoto H, Kamada T. Biochem Biophys Res Commun. 1994;204:468–474. doi: 10.1006/bbrc.1994.2483. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi T, Tanaka M, Inazawa J, Abe T, Suda T, Nagata S. Int Immunol. 1994;6:1567–1574. doi: 10.1093/intimm/6.10.1567. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka M, Suda T, Takahashi T, Nagata S. EMBO J. 1995;14:1129–1135. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicoletti I, Migliorati G, Pagliacci M C, Grignani F, Riccardi C. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 24.Mosmann T. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka M, Suda T, Haze K, Nakamura N, Sato K, Kimura F, Motoyoshi K, Mizuki M, Tagawa S, Ohga S, Hatake K, Drummond A H, Nagata S. Nat Med. 1996;2:317–322. doi: 10.1038/nm0396-317. [DOI] [PubMed] [Google Scholar]
- 26.Yonehara S, Ishii A, Yonehara M. J Exp Med. 1989;169:1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weis M, Schlegel J, Kass G E N, Holmström T H, Peters I, Eriksson J, Orrenius S, Chow S C. Exp Cell Res. 1995;219:699–708. doi: 10.1006/excr.1995.1281. [DOI] [PubMed] [Google Scholar]
- 28.Nagata S. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 29.Henkart P A. Immunity. 1994;1:343–346. doi: 10.1016/1074-7613(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 30.Berke G. Cell. 1995;81:9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- 31.Doherty P C. Cell. 1993;75:607–612. doi: 10.1016/0092-8674(93)90480-e. [DOI] [PubMed] [Google Scholar]
- 32.Abbas A K. Cell. 1996;84:655–657. doi: 10.1016/s0092-8674(00)81042-9. [DOI] [PubMed] [Google Scholar]
- 33.Kubota Y, Sunouchi K, Ono M, Sawada T, Muto T. Dis Colon Rectum. 1992;35:645–650. doi: 10.1007/BF02053754. [DOI] [PubMed] [Google Scholar]
- 34.Underwood J C. Br J Cancer. 1974;30:538–548. doi: 10.1038/bjc.1974.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vose B M, Moore M. Semin Hematol. 1985;22:27–40. [PubMed] [Google Scholar]
- 36.Seino K, Kayagaki N, Okumura K, Yagita H. Nat Med. 1997;2:165–170. doi: 10.1038/nm0297-165. [DOI] [PubMed] [Google Scholar]
- 37.Weiss L, Grundmann E, Torhorst J, Hartveit F, Moberg I, Eder M, Fenoglio-Preiser C M, Napier J, Horne C H, Lopez M J, Shaw-Dunn R I, Sugar J, Davies J D, Day D W, Harlos J P. J Pathol. 1986;150:195–203. doi: 10.1002/path.1711500308. [DOI] [PubMed] [Google Scholar]
- 38.Hanahan D, Folkman J. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 39.Fidler I J. Cancer Res. 1990;50:6130–6138. [PubMed] [Google Scholar]
- 40.Liotta L A, Steeg P S, Stetler-Stevenson W G. Cell. 1991;64:327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- 41.Kuo T H, Kubota T, Watanabe M, Furukawa T, Teramoto T, Ishibiki K, Kitajima M, Moossa A R, Penman S, Hoffman R M. Proc Natl Acad Sci USA. 1995;92:12085–12089. doi: 10.1073/pnas.92.26.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spremuli E N, Dexter D L. J Clin Oncol. 1983;1:496–509. doi: 10.1200/JCO.1983.1.8.496. [DOI] [PubMed] [Google Scholar]
- 43.Fidler I J. Cytometry. 1989;10:673–680. doi: 10.1002/cyto.990100602. [DOI] [PubMed] [Google Scholar]
- 44.Nigam A K, Pignatelli M, Boulos P B. Gut. 1994;35:996–1000. doi: 10.1136/gut.35.7.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galle P R, Hofmann W J, Walczak H, Schaller H, Otto G, Stremmel W, Krammer P H, Runkel L. J Exp Med. 1995;182:1223–1230. doi: 10.1084/jem.182.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Nature (London) 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 47.Ni R, Tomita Y, Ichihara A, Ishimura K, Ogasawara J, Nagata S. Exp Cell Res. 1994;215:332–337. doi: 10.1006/excr.1994.1349. [DOI] [PubMed] [Google Scholar]
- 48.Owen-Schaub L B, Radinsky R, Kruzel E, Berry K, Yonehara S. Cancer Res. 1994;54:1580–1586. [PubMed] [Google Scholar]