Abstract
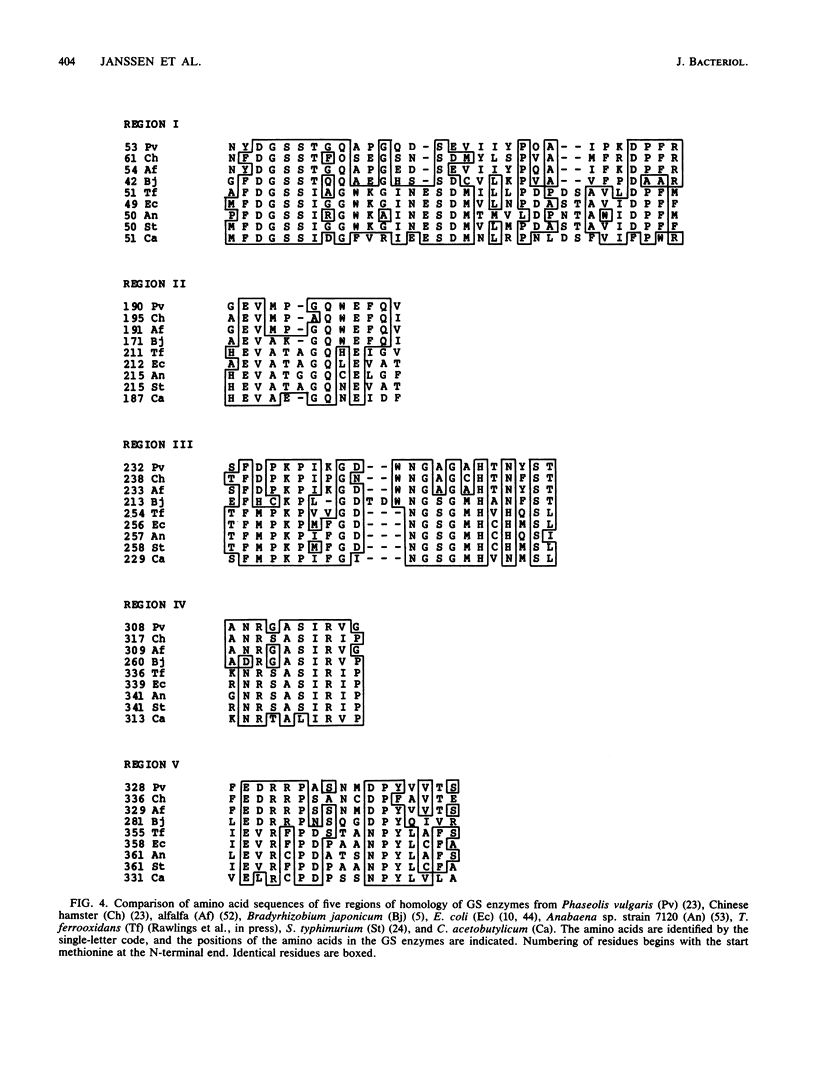
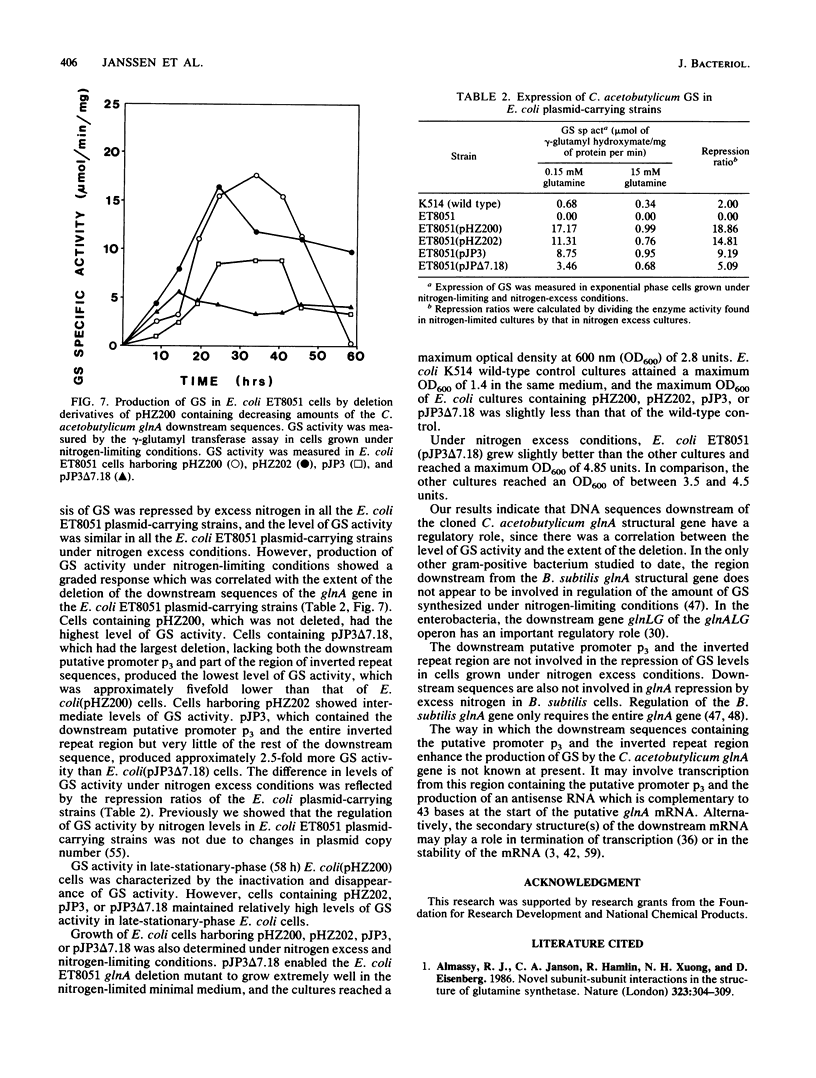
The nucleotide sequence of a 2.0-kilobase DNA segment containing the Clostridium acetobutylicum glnA gene was determined. The upstream region of the glnA gene contained two putative extended promoter consensus sequences (p1 and p2), characteristic of gram-positive bacteria. A third putative extended gram-positive promoter consensus sequence (p3), oriented towards the glnA gene, was detected downstream of the structural gene. The sequences containing the proposed promoter regions p1 and p2 or p3 were shown to have promoter activity by subcloning into promoter probe vectors. The complete amino acid sequence (444 residues) of the C. acetobutylicum glutamine synthetase (GS) was deduced, and comparisons were made with the reported amino acid sequences of GS from other organisms. To determine whether the putative promoter p3 and a downstream region with an extensive stretch of inverted repeat sequences were involved in regulation of C. acetobutylicum glnA gene expression by nitrogen in Escherichia coli, deletion plasmids were constructed lacking p3 and various downstream sequences. Deletion of the putative promoter p3 and downstream inverted repeat sequences affected the regulation of GS and reduced the levels of GS approximately fivefold under nitrogen-limiting conditions but did not affect the repression of GS levels in cells grown under nitrogen-excess conditions.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almassy R. J., Janson C. A., Hamlin R., Xuong N. H., Eisenberg D. Novel subunit-subunit interactions in the structure of glutamine synthetase. 1986 Sep 25-Oct 1Nature. 323(6086):304–309. doi: 10.1038/323304a0. [DOI] [PubMed] [Google Scholar]
- Andersen J., Delihas N., Ikenaka K., Green P. J., Pines O., Ilercil O., Inouye M. The isolation and characterization of RNA coded by the micF gene in Escherichia coli. Nucleic Acids Res. 1987 Mar 11;15(5):2089–2101. doi: 10.1093/nar/15.5.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belasco J. G., Beatty J. T., Adams C. W., von Gabain A., Cohen S. N. Differential expression of photosynthesis genes in R. capsulata results from segmental differences in stability within the polycistronic rxcA transcript. Cell. 1985 Jan;40(1):171–181. doi: 10.1016/0092-8674(85)90320-4. [DOI] [PubMed] [Google Scholar]
- Belin D., Mudd E. A., Prentki P., Yi-Yi Y., Krisch H. M. Sense and antisense transcription of bacteriophage T4 gene 32. Processing and stability of the mRNAs. J Mol Biol. 1987 Mar 20;194(2):231–243. doi: 10.1016/0022-2836(87)90371-8. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Chen K. C., Chen J. S., Johnson J. L. Structural features of multiple nifH-like sequences and very biased codon usage in nitrogenase genes of Clostridium pasteurianum. J Bacteriol. 1986 Apr;166(1):162–172. doi: 10.1128/jb.166.1.162-172.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo G., Villafranca J. J. Amino acid sequence of Escherichia coli glutamine synthetase deduced from the DNA nucleotide sequence. J Biol Chem. 1986 Aug 15;261(23):10587–10591. [PubMed] [Google Scholar]
- Corfield V. A., Reid S. J., Bodmer J., Thomson J. A. A modified protoplast-regeneration protocol facilitating the detection of cloned exoenzyme genes in Bacillus subtilis. Gene. 1984 Oct;30(1-3):17–22. doi: 10.1016/0378-1119(84)90100-8. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Darrow R. A., Knotts R. R. Two forms of glutamine synthetase in free-living root-nodule bacteria. Biochem Biophys Res Commun. 1977 Sep 23;78(2):554–559. doi: 10.1016/0006-291x(77)90214-5. [DOI] [PubMed] [Google Scholar]
- Dautry-Varsat A., Cohen G. N., Stadtman E. R. Some properties of Escherichia coli glutamine synthetase after limited proteolysis by subtilisin. J Biol Chem. 1979 Apr 25;254(8):3124–3128. [PubMed] [Google Scholar]
- Duvall E. J., Williams D. M., Lovett P. S., Rudolph C., Vasantha N., Guyer M. Chloramphenicol-inducible gene expression in Bacillus subtilis. Gene. 1983 Oct;24(2-3):171–177. doi: 10.1016/0378-1119(83)90077-x. [DOI] [PubMed] [Google Scholar]
- Filser D. M., Moscatelli C., Lamberti A., Vincze E., Guida M., Salzano G., Iaccarino M. Characterization and cloning of two Rhizobium leguminosarum genes coding for glutamine synthetase activities. J Gen Microbiol. 1986 Sep;132(9):2561–2569. doi: 10.1099/00221287-132-9-2561. [DOI] [PubMed] [Google Scholar]
- Fisher R., Tuli R., Haselkorn R. A cloned cyanobacterial gene for glutamine synthetase functions in Escherichia coli, but the enzyme is not adenylylated. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3393–3397. doi: 10.1073/pnas.78.6.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt C., Oliver J. E., Forde B. G., Saarelainen R., Miflin B. J. Primary structure and differential expression of glutamine synthetase genes in nodules, roots and leaves of Phaseolus vulgaris. EMBO J. 1986 Jul;5(7):1429–1435. doi: 10.1002/j.1460-2075.1986.tb04379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough N. M., Webb E. A., Cory S., Adams J. M. Molecular cloning of seven mouse immunoglobulin kappa chain messenger ribonucleic acids. Biochemistry. 1980 Jun 10;19(12):2702–2710. doi: 10.1021/bi00553a026. [DOI] [PubMed] [Google Scholar]
- Graves M. C., Rabinowitz J. C. In vivo and in vitro transcription of the Clostridium pasteurianum ferredoxin gene. Evidence for "extended" promoter elements in gram-positive organisms. J Biol Chem. 1986 Aug 25;261(24):11409–11415. [PubMed] [Google Scholar]
- Green P. J., Pines O., Inouye M. The role of antisense RNA in gene regulation. Annu Rev Biochem. 1986;55:569–597. doi: 10.1146/annurev.bi.55.070186.003033. [DOI] [PubMed] [Google Scholar]
- Hayward B. E., Hussain A., Wilson R. H., Lyons A., Woodcock V., McIntosh B., Harris T. J. The cloning and nucleotide sequence of cDNA for an amplified glutamine synthetase gene from the Chinese hamster. Nucleic Acids Res. 1986 Jan 24;14(2):999–1008. doi: 10.1093/nar/14.2.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson C. A., Kayne P. S., Almassy R. J., Grunstein M., Eisenberg D. Sequence of glutamine synthetase from Salmonella typhimurium and implications for the protein structure. Gene. 1986;46(2-3):297–300. doi: 10.1016/0378-1119(86)90415-4. [DOI] [PubMed] [Google Scholar]
- Joliff G., Béguin P., Aubert J. P. Nucleotide sequence of the cellulase gene celD encoding endoglucanase D of Clostridium thermocellum. Nucleic Acids Res. 1986 Nov 11;14(21):8605–8613. doi: 10.1093/nar/14.21.8605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. T., Woods D. R. Acetone-butanol fermentation revisited. Microbiol Rev. 1986 Dec;50(4):484–524. doi: 10.1128/mr.50.4.484-524.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lévi-Meyrueis C., Fodor K., Schaeffer P. Polyethyleneglycol-induced transformation of Bacillus subtilis protoplasts by bacterial chromosomal DNA. Mol Gen Genet. 1980;179(3):589–594. doi: 10.1007/BF00271749. [DOI] [PubMed] [Google Scholar]
- Magasanik B. Genetic control of nitrogen assimilation in bacteria. Annu Rev Genet. 1982;16:135–168. doi: 10.1146/annurev.ge.16.120182.001031. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mongkolsuk S., Chiang Y. W., Reynolds R. B., Lovett P. S. Restriction fragments that exert promoter activity during postexponential growth of Bacillus subtilis. J Bacteriol. 1983 Sep;155(3):1399–1406. doi: 10.1128/jb.155.3.1399-1406.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe D. M., Noyes C. M., Griffith M. J., Lundblad R. L., Kingdon H. S. Structural and enzymatic properties of Escherichia coli glutamine synthetase subjected to limited proteolysis. Curr Top Cell Regul. 1985;27:361–372. doi: 10.1016/b978-0-12-152827-0.50038-4. [DOI] [PubMed] [Google Scholar]
- Moon K., Smith E. L. Sequence of bovine liver glutamate dehydrogenase. 8. Peptides produced by specific chemical cleavages; the complete sequence of the protein. J Biol Chem. 1973 May 10;248(9):3082–3088. [PubMed] [Google Scholar]
- Morgan W. D., Bear D. G., Litchman B. L., von Hippel P. H. RNA sequence and secondary structure requirements for rho-dependent transcription termination. Nucleic Acids Res. 1985 May 24;13(10):3739–3754. doi: 10.1093/nar/13.10.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima R., Imanaka T., Aiba S. Nucleotide sequence of the Bacillus stearothermophilus alpha-amylase gene. J Bacteriol. 1985 Jul;163(1):401–406. doi: 10.1128/jb.163.1.401-406.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Freundlich M. Mechanism for the autogenous control of the crp operon: transcriptional inhibition by a divergent RNA transcript. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5000–5004. doi: 10.1073/pnas.83.14.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahel G., Tyler B. A new glnA-linked regulatory gene for glutamine synthetase in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4544–4548. doi: 10.1073/pnas.76.9.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido D., Jiménez A. Optimization of gene expression in Streptomyces lividans by a transcription terminator. Nucleic Acids Res. 1987 May 26;15(10):4227–4240. doi: 10.1093/nar/15.10.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasched I., Jörnvall H., Sund H. Studies of glutamate dehydrogenase. Identification of an amino group involved in the substrate binding. Eur J Biochem. 1974 Feb 1;41(3):603–606. doi: 10.1111/j.1432-1033.1974.tb03302.x. [DOI] [PubMed] [Google Scholar]
- Rocha M., Vázquez M., Garciarrubio A., Covarrubias A. A. Nucleotide sequence of the glnA-glnL intercistronic region of Escherichia coli. Gene. 1985;37(1-3):91–99. doi: 10.1016/0378-1119(85)90261-6. [DOI] [PubMed] [Google Scholar]
- Salser W. Globin mRNA sequences: analysis of base pairing and evolutionary implications. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):985–1002. doi: 10.1101/sqb.1978.042.01.099. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier H. J., Fisher S. H., Sonenshein A. L. Regulation of expression from the glnA promoter of Bacillus subtilis requires the glnA gene product. Proc Natl Acad Sci U S A. 1985 May;82(10):3375–3379. doi: 10.1073/pnas.82.10.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier H. J., Sonenshein A. L. Altered regulation of the glnA gene in glutamine synthetase mutants of Bacillus subtilis. J Bacteriol. 1986 Jul;167(1):35–43. doi: 10.1128/jb.167.1.35-43.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira S. K., Chou J., Richaud F. V., Casadaban M. J. New versatile plasmid vectors for expression of hybrid proteins coded by a cloned gene fused to lacZ gene sequences encoding an enzymatically active carboxy-terminal portion of beta-galactosidase. Gene. 1983 Nov;25(1):71–82. doi: 10.1016/0378-1119(83)90169-5. [DOI] [PubMed] [Google Scholar]
- Shapiro B. M., Stadtman E. R. 5'-adenylyl-O-tyrosine. The novel phosphodiester residue of adenylylated glutamine synthetase from Escherichia coli. J Biol Chem. 1968 Jul 10;243(13):3769–3771. [PubMed] [Google Scholar]
- Tyler B. Regulation of the assimilation of nitrogen compounds. Annu Rev Biochem. 1978;47:1127–1162. doi: 10.1146/annurev.bi.47.070178.005403. [DOI] [PubMed] [Google Scholar]
- Usdin K. P., Zappe H., Jones D. T., Woods D. R. Cloning, Expression, and Purification of Glutamine Synthetase from Clostridium acetobutylicum. Appl Environ Microbiol. 1986 Sep;52(3):413–419. doi: 10.1128/aem.52.3.413-419.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. M., Duvall E. J., Lovett P. S. Cloning restriction fragments that promote expression of a gene in Bacillus subtilis. J Bacteriol. 1981 Jun;146(3):1162–1165. doi: 10.1128/jb.146.3.1162-1165.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H. C., Chang S. Identification of a positive retroregulator that stabilizes mRNAs in bacteria. Proc Natl Acad Sci U S A. 1986 May;83(10):3233–3237. doi: 10.1073/pnas.83.10.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabeau M., Stanley K. K. Enhanced expression of cro-beta-galactosidase fusion proteins under the control of the PR promoter of bacteriophage lambda. EMBO J. 1982;1(10):1217–1224. doi: 10.1002/j.1460-2075.1982.tb00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



