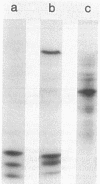
Abstract
Selenium is a constituent in Escherichia coli of the anaerobic enzyme formate dehydrogenase in the form of selenocysteine. Selenium is also present in the tRNA of E. coli in the modified base 5-methylaminomethyl-2-selenouracil (mnm5Se2U). The pathways of bacterial selenium metabolism are largely uncharacterized, and it is unclear whether nonspecific reactions in the sulfur metabolic pathways may be involved. We demonstrated that sulfur metabolic pathway mutants retain a wild-type pattern of selenium incorporation, indicating that selenite (SeO32-) is metabolized entirely via selenium-specific pathways. To investigate the function of mnm5Se2U, we isolated a mutant which is unable to incorporate selenium into tRNA. This strain was obtained by isolating mutants lacking formate dehydrogenase activity and then screening for the inability to metabolize selenium. This phenotype is the result of a recessive mutation which appears to map in the general region of 21 min on the Salmonella typhimurium chromosome. A mutation in this gene, selA, thus has a pleiotropic effect of eliminating selenium incorporation into both protein and tRNA. The selA mutant appears to be blocked in a step of selenium metabolism after reduction, such as in the actual selenium insertion process. We showed that the absence of selenium incorporation into suppressor tRNA reduces the efficiency of suppression of nonsense codons in certain contexts and when wobble base pairing is required. Thus, one function of mnm5Se2U in tRNA may be in codon-anticodon interactions.
Full text
PDF
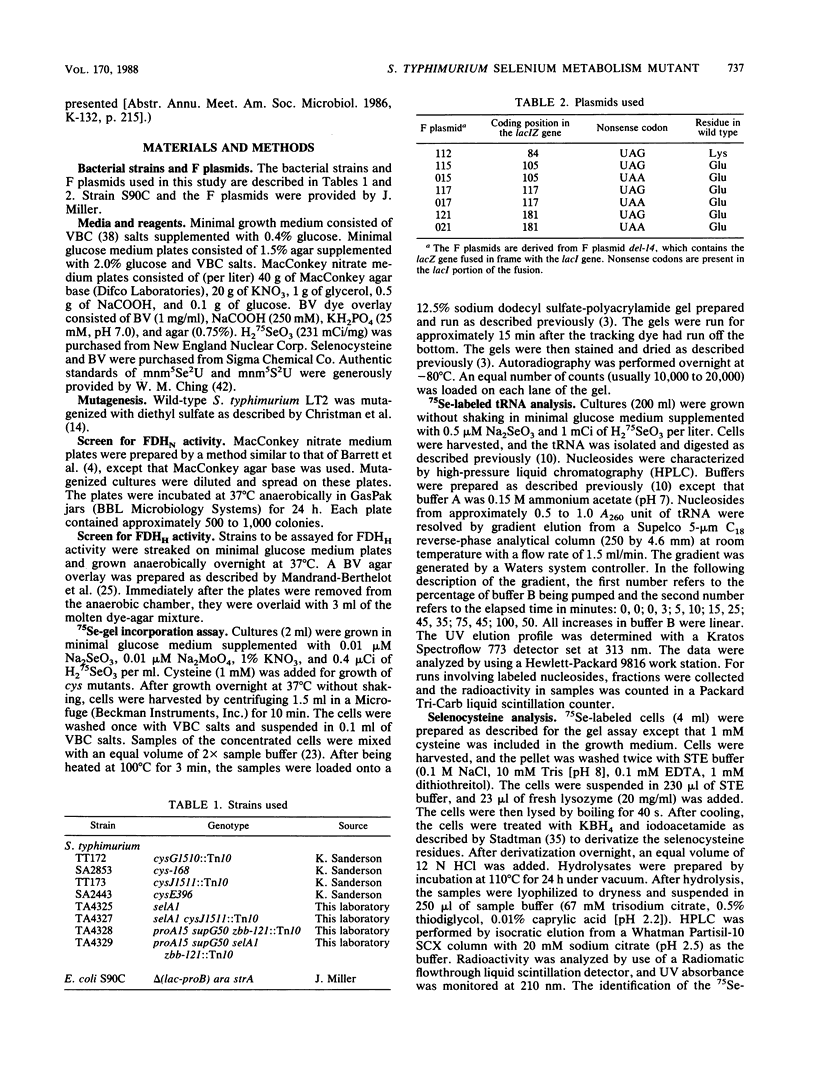

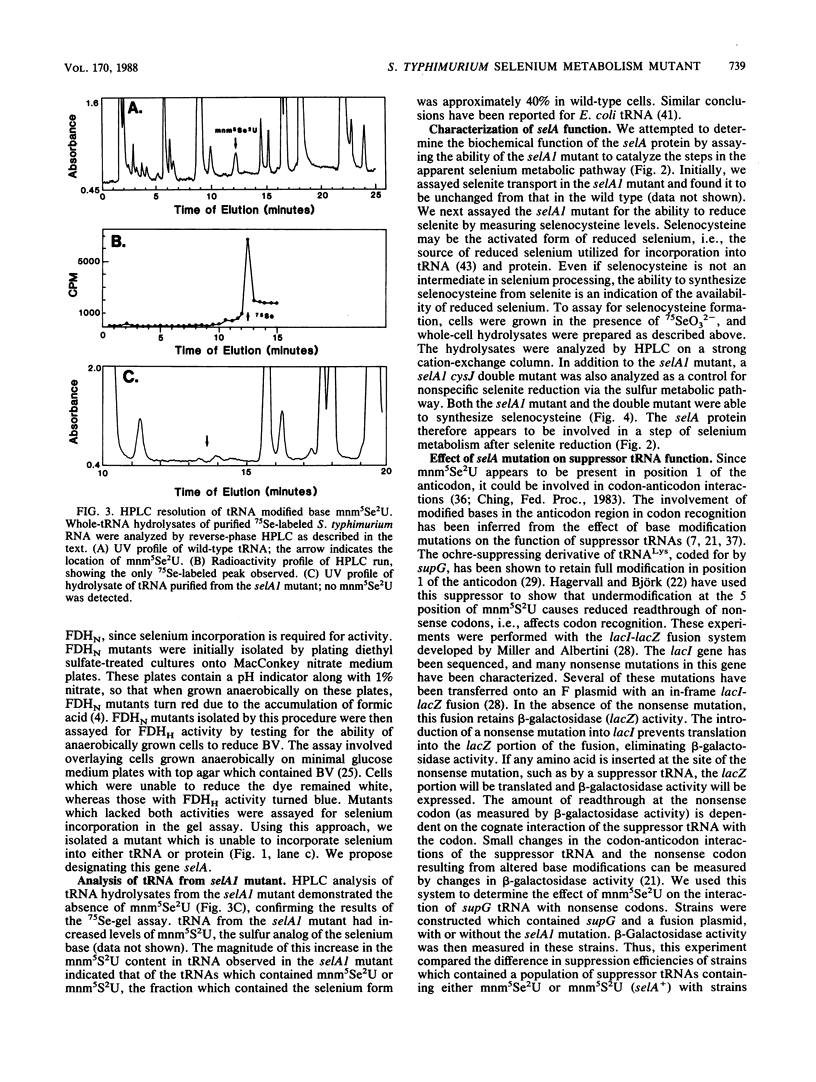
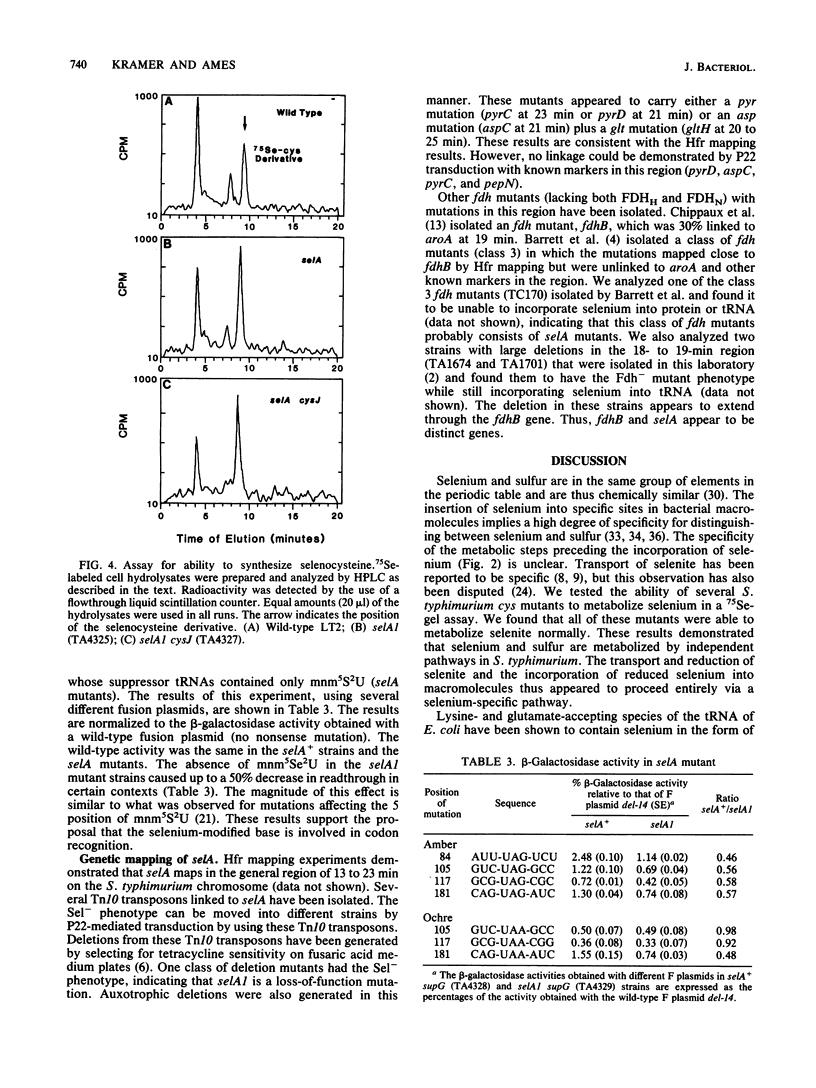



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrell J. W., Kaufman E. E., Lipsett M. N. The biosynthesis of 4-thiouridylate. Separation and purification of two enzymes in the transfer ribonucleic acid-sulfurtransferase system. J Biol Chem. 1971 Jan 25;246(2):294–301. [PubMed] [Google Scholar]
- Alper M. D., Ames B. N. Positive selection of mutants with deletions of the gal-chl region of the Salmonella chromosome as a screening procedure for mutagens that cause deletions. J Bacteriol. 1975 Jan;121(1):259–266. doi: 10.1128/jb.121.1.259-266.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Barrett E. L., Jackson C. E., Fukumoto H. T., Chang G. W. Formate dehydrogenase mutants of Salmonella typhimurium: a new medium for their isolation and new mutant classes. Mol Gen Genet. 1979;177(1):95–101. doi: 10.1007/BF00267258. [DOI] [PubMed] [Google Scholar]
- Björk G. R., Kjellin-Stråby K. Escherichia coli mutants with defects in the biosynthesis of 5-methylaminomethyl-2-thio-uridine or 1-methylguanosine in their tRNA. J Bacteriol. 1978 Feb;133(2):508–517. doi: 10.1128/jb.133.2.508-517.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouadloun F., Srichaiyo T., Isaksson L. A., Björk G. R. Influence of modification next to the anticodon in tRNA on codon context sensitivity of translational suppression and accuracy. J Bacteriol. 1986 Jun;166(3):1022–1027. doi: 10.1128/jb.166.3.1022-1027.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. A., Shrift A. Assimilation of selenate and selenite by Salmonella typhimurium. Can J Microbiol. 1980 Jun;26(6):671–675. doi: 10.1139/m80-117. [DOI] [PubMed] [Google Scholar]
- Brown T. A., Shrift A. Selective assimilation of selenite by Escherichia coli. Can J Microbiol. 1982 Mar;28(3):307–310. doi: 10.1139/m82-045. [DOI] [PubMed] [Google Scholar]
- Buck M., Connick M., Ames B. N. Complete analysis of tRNA-modified nucleosides by high-performance liquid chromatography: the 29 modified nucleosides of Salmonella typhimurium and Escherichia coli tRNA. Anal Biochem. 1983 Feb 15;129(1):1–13. doi: 10.1016/0003-2697(83)90044-1. [DOI] [PubMed] [Google Scholar]
- Chambers I., Frampton J., Goldfarb P., Affara N., McBain W., Harrison P. R. The structure of the mouse glutathione peroxidase gene: the selenocysteine in the active site is encoded by the 'termination' codon, TGA. EMBO J. 1986 Jun;5(6):1221–1227. doi: 10.1002/j.1460-2075.1986.tb04350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W. M., Tsai L., Wittwer A. J. Selenium-containing transfer RNAs. Curr Top Cell Regul. 1985;27:497–507. doi: 10.1016/b978-0-12-152827-0.50050-5. [DOI] [PubMed] [Google Scholar]
- Chippaux M., Pascal M. C., Casse F. Formate hydrogenlyase system in Salmonella typhimurium LT2. Eur J Biochem. 1977 Jan 3;72(1):149–155. doi: 10.1111/j.1432-1033.1977.tb11234.x. [DOI] [PubMed] [Google Scholar]
- Christman M. F., Morgan R. W., Jacobson F. S., Ames B. N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985 Jul;41(3):753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- Cox J. C., Edwards E. S., DeMoss J. A. Resolution of distinct selenium-containing formate dehydrogenases from Escherichia coli. J Bacteriol. 1981 Mar;145(3):1317–1324. doi: 10.1128/jb.145.3.1317-1324.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elseviers D., Petrullo L. A., Gallagher P. J. Novel E. coli mutants deficient in biosynthesis of 5-methylaminomethyl-2-thiouridine. Nucleic Acids Res. 1984 Apr 25;12(8):3521–3534. doi: 10.1093/nar/12.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch H. G., Lester R. L. The purification and properties of formate dehydrogenase and nitrate reductase from Escherichia coli. J Biol Chem. 1975 Sep 10;250(17):6693–6705. [PubMed] [Google Scholar]
- Gauss D. H., Sprinzl M. Compilation of tRNA sequences. Nucleic Acids Res. 1983 Jan 11;11(1):r1–53. [PMC free article] [PubMed] [Google Scholar]
- Grosjean H., Chantrenne H. On codon- anticodon interactions. Mol Biol Biochem Biophys. 1980;32:347–367. doi: 10.1007/978-3-642-81503-4_27. [DOI] [PubMed] [Google Scholar]
- Hagervall T. G., Björk G. R. Undermodification in the first position of the anticodon of supG-tRNA reduces translational efficiency. Mol Gen Genet. 1984;196(2):194–200. doi: 10.1007/BF00328050. [DOI] [PubMed] [Google Scholar]
- Kredich N. M. Regulation of L-cysteine biosynthesis in Salmonella typhimurium. I. Effects of growth of varying sulfur sources and O-acetyl-L-serine on gene expression. J Biol Chem. 1971 Jun 10;246(11):3474–3484. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindblow-Kull C., Kull F. J., Shrift A. Single transporter for sulfate, selenate, and selenite in Escherichia coli K-12. J Bacteriol. 1985 Sep;163(3):1267–1269. doi: 10.1128/jb.163.3.1267-1269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinus M. G., Morris N. R., Söll D., Kwong T. C. Isolation and partial characterization of three Escherichia coli mutants with altered transfer ribonucleic acid methylases. J Bacteriol. 1975 Apr;122(1):257–265. doi: 10.1128/jb.122.1.257-265.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. H., Albertini A. M. Effects of surrounding sequence on the suppression of nonsense codons. J Mol Biol. 1983 Feb 15;164(1):59–71. doi: 10.1016/0022-2836(83)90087-6. [DOI] [PubMed] [Google Scholar]
- Prather N. E., Mims B. H., Murgola E. J. supG and supL in Escherichia coli code for mutant lysine tRNAs+. Nucleic Acids Res. 1983 Dec 10;11(23):8283–8286. doi: 10.1093/nar/11.23.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy C. C., Massaro E. J. Biochemistry of selenium: a brief overview. Fundam Appl Toxicol. 1983 Sep-Oct;3(5):431–436. doi: 10.1016/s0272-0590(83)80017-7. [DOI] [PubMed] [Google Scholar]
- Ryals J., Hsu R. Y., Lipsett M. N., Bremer H. Isolation of single-site Escherichia coli mutants deficient in thiamine and 4-thiouridine syntheses: identification of a nuvC mutant. J Bacteriol. 1982 Aug;151(2):899–904. doi: 10.1128/jb.151.2.899-904.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpaer E. G. Constraints on codon context in Escherichia coli genes. Their possible role in modulating the efficiency of translation. J Mol Biol. 1986 Apr 20;188(4):555–564. doi: 10.1016/s0022-2836(86)80005-5. [DOI] [PubMed] [Google Scholar]
- Stadtman T. C. New biologic functions--selenium-dependent nucleic acids and proteins. Fundam Appl Toxicol. 1983 Sep-Oct;3(5):420–423. doi: 10.1016/s0272-0590(83)80015-3. [DOI] [PubMed] [Google Scholar]
- Stadtman T. C. Occurrence and characterization of selenocysteine in proteins. Methods Enzymol. 1984;107:576–581. doi: 10.1016/0076-6879(84)07041-5. [DOI] [PubMed] [Google Scholar]
- Sullivan M. A., Cannon J. F., Webb F. H., Bock R. M. Antisuppressor mutation in Escherichia coli defective in biosynthesis of 5-methylaminomethyl-2-thiouridine. J Bacteriol. 1985 Jan;161(1):368–376. doi: 10.1128/jb.161.1.368-376.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- WILSON L. G., BANDURSKI R. S. Enzymatic reactions involving sulfate, sulfite, selenate, and molybdate. J Biol Chem. 1958 Oct;233(4):975–981. [PubMed] [Google Scholar]
- Wittwer A. J. Specific incorporation of selenium into lysine- and glutamate- accepting tRNAs from Escherichia coli. J Biol Chem. 1983 Jul 25;258(14):8637–8641. [PubMed] [Google Scholar]
- Wittwer A. J., Stadtman T. C. Biosynthesis of 5-methylaminomethyl-2-selenouridine, a naturally occurring nucleoside in Escherichia coli tRNA. Arch Biochem Biophys. 1986 Aug 1;248(2):540–550. doi: 10.1016/0003-9861(86)90507-2. [DOI] [PubMed] [Google Scholar]
- Wittwer A. J., Tsai L., Ching W. M., Stadtman T. C. Identification and synthesis of a naturally occurring selenonucleoside in bacterial tRNAs: 5-[(methylamino)methyl]-2-selenouridine. Biochemistry. 1984 Sep 25;23(20):4650–4655. doi: 10.1021/bi00315a021. [DOI] [PubMed] [Google Scholar]
- Young P. A., Kaiser I. I. Aminoacylation of Escherichia coli cysteine tRNA by selenocysteine. Arch Biochem Biophys. 1975 Dec;171(2):483–489. doi: 10.1016/0003-9861(75)90057-0. [DOI] [PubMed] [Google Scholar]
- Zinoni F., Birkmann A., Stadtman T. C., Böck A. Nucleotide sequence and expression of the selenocysteine-containing polypeptide of formate dehydrogenase (formate-hydrogen-lyase-linked) from Escherichia coli. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4650–4654. doi: 10.1073/pnas.83.13.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]