Abstract
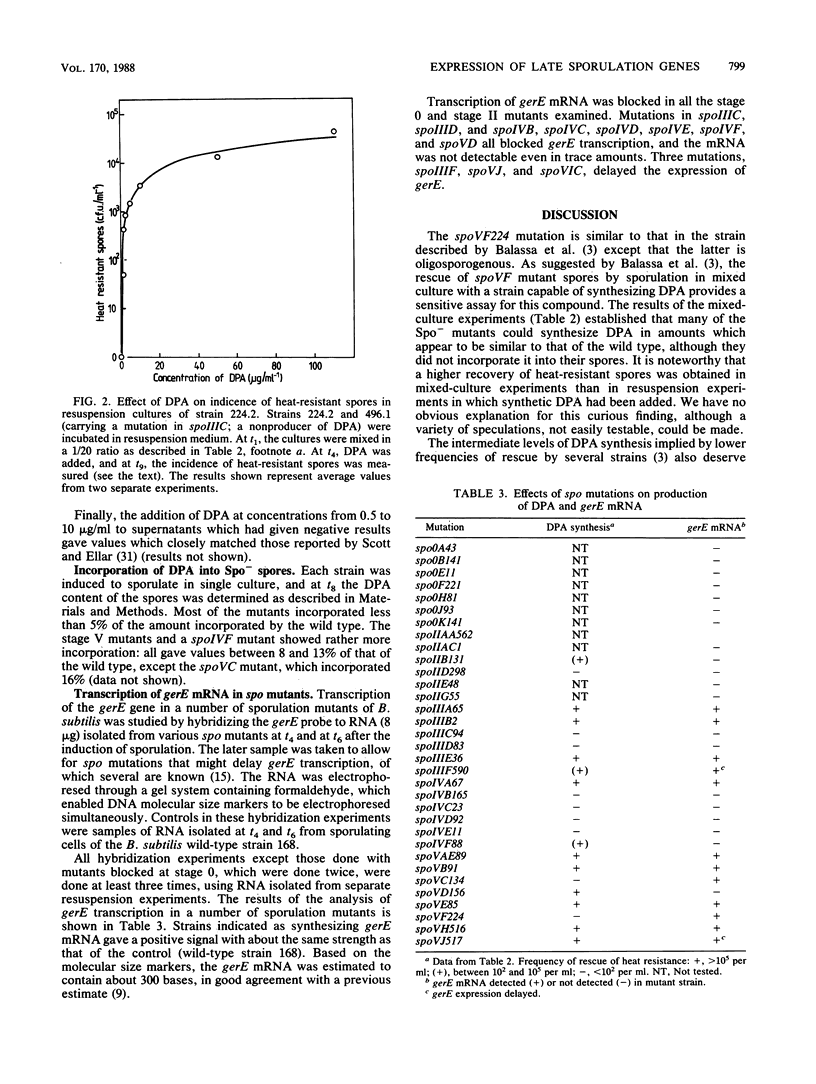
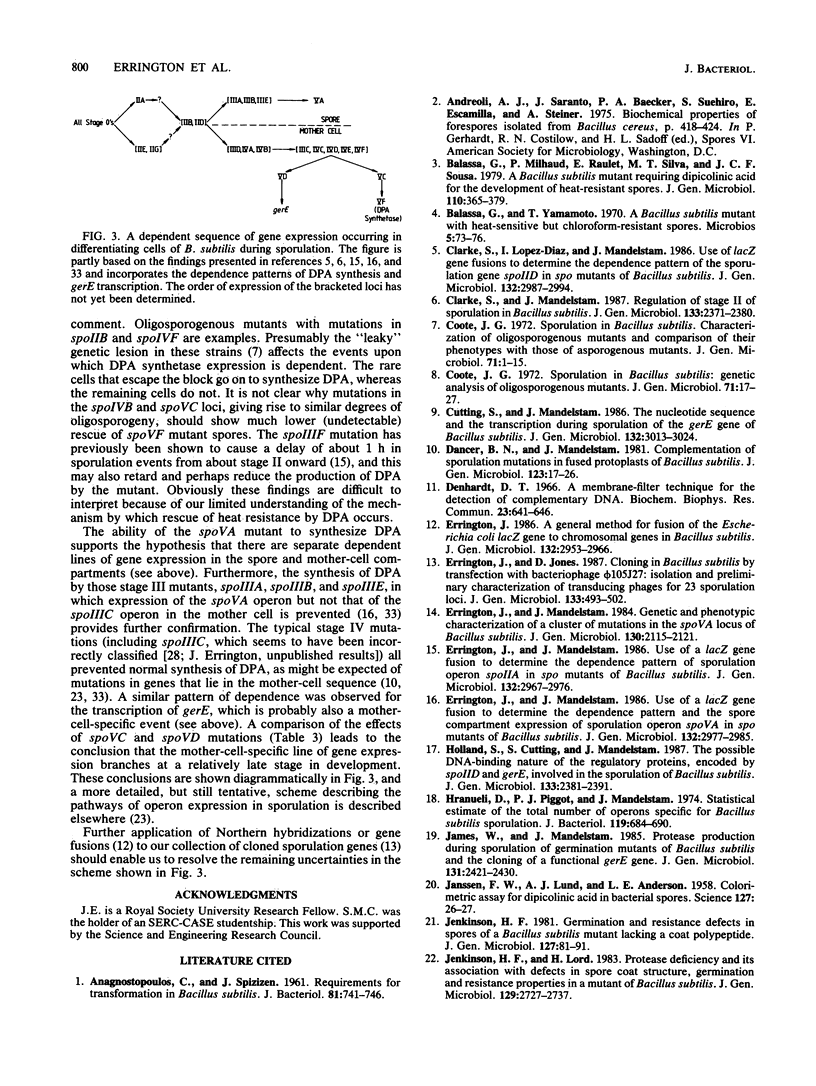
We measured the synthesis of dipicolinic acid (DPA) during sporulation in spo mutants of Bacillus subtilis by a sensitive biological assay based on cross-feeding of a spoVF mutant strain and also chemically. Many spo mutations, including several that block sporulation at stage III, did not prevent synthesis of DPA but instead prevented its incorporation into the spore. In general, strains with mutations in loci that are expressed in the spore compartment synthesized DPA, whereas strains with mutations in most of the loci that are expressed in the mother-cell compartment did not. Transcription of the gerE gene, as measured by DNA-RNA hybridization, followed a dependence pattern very similar to that of DPA synthesis. However, the dependence patterns of the two operons show that at about stage IV of sporulation there is a branch in the sequence of operon expression in the mother cell. One branch leads through spoVC to synthesis of DPA synthetase, and the other leads through spoVD to expression of gerE.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balassa G., Milhaud P., Raulet E., Silva M. T., Sousa J. C. A Bacillus subtilis mutant requiring dipicolinic acid for the development of heat-resistant spores. J Gen Microbiol. 1979 Feb;110(2):365–379. doi: 10.1099/00221287-110-2-365. [DOI] [PubMed] [Google Scholar]
- Clarke S., Lopez-Diaz I., Mandelstam J. Use of lacZ gene fusions to determine the dependence pattern of the sporulation gene spoIID in spo mutants of Bacillus subtilis. J Gen Microbiol. 1986 Nov;132(11):2987–2994. doi: 10.1099/00221287-132-11-2987. [DOI] [PubMed] [Google Scholar]
- Clarke S., Mandelstam J. Regulation of stage II of sporulation in Bacillus subtilis. J Gen Microbiol. 1987 Sep;133(9):2371–2380. doi: 10.1099/00221287-133-9-2371. [DOI] [PubMed] [Google Scholar]
- Coote J. G. Sporulation in Bacillus subtilis. Characterization of oligosporogenous mutants and comparison of their phenotypes with those of asporogenous mutants. J Gen Microbiol. 1972 Jun;71(1):1–15. doi: 10.1099/00221287-71-1-1. [DOI] [PubMed] [Google Scholar]
- Coote J. G. Sporulation in Bacillus subtilis. Genetic analysis of oligosporogenous mutants. J Gen Microbiol. 1972 Jun;71(1):17–27. doi: 10.1099/00221287-71-1-17. [DOI] [PubMed] [Google Scholar]
- Cutting S., Mandelstam J. The nucleotide sequence and the transcription during sporulation of the gerE gene of Bacillus subtilis. J Gen Microbiol. 1986 Nov;132(11):3013–3024. doi: 10.1099/00221287-132-11-3013. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Errington J. A general method for fusion of the Escherichia coli lacZ gene to chromosomal genes in Bacillus subtilis. J Gen Microbiol. 1986 Nov;132(11):2953–2966. doi: 10.1099/00221287-132-11-2953. [DOI] [PubMed] [Google Scholar]
- Errington J., Jones D. Cloning in Bacillus subtilis by transfection with bacteriophage vector phi 105J27: isolation and preliminary characterization of transducing phages for 23 sporulation loci. J Gen Microbiol. 1987 Mar;133(3):493–502. doi: 10.1099/00221287-133-3-493. [DOI] [PubMed] [Google Scholar]
- Errington J., Mandelstam J. Genetic and phenotypic characterization of a cluster of mutations in the spoVA locus of Bacillus subtilis. J Gen Microbiol. 1984 Aug;130(8):2115–2121. doi: 10.1099/00221287-130-8-2115. [DOI] [PubMed] [Google Scholar]
- Errington J., Mandelstam J. Use of a lacZ gene fusion to determine the dependence pattern and the spore compartment expression of sporulation operon spoVA in spo mutants of Bacillus subtilis. J Gen Microbiol. 1986 Nov;132(11):2977–2985. doi: 10.1099/00221287-132-11-2977. [DOI] [PubMed] [Google Scholar]
- Errington J., Mandelstam J. Use of a lacZ gene fusion to determine the dependence pattern of sporulation operon spoIIA in spo mutants of Bacillus subtilis. J Gen Microbiol. 1986 Nov;132(11):2967–2976. doi: 10.1099/00221287-132-11-2967. [DOI] [PubMed] [Google Scholar]
- Holland S. K., Cutting S., Mandelstam J. The possible DNA-binding nature of the regulatory proteins, encoded by spoIID and gerE, involved in the sporulation of Bacillus subtilis. J Gen Microbiol. 1987 Sep;133(9):2381–2391. doi: 10.1099/00221287-133-9-2381. [DOI] [PubMed] [Google Scholar]
- Hranueli D., Piggot P. J., Mandelstam J. Statistical estimate of the total number of operons specific for Bacillus subtilis sporulation. J Bacteriol. 1974 Sep;119(3):684–690. doi: 10.1128/jb.119.3.684-690.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- James W., Mandelstam J. Protease production during sporulation of germination mutants of Bacillus subtilis and the cloning of a functional gerE gene. J Gen Microbiol. 1985 Sep;131(9):2421–2430. doi: 10.1099/00221287-131-9-2421. [DOI] [PubMed] [Google Scholar]
- Mandelstam J., Errington J. Dependent sequences of gene expression controlling spore formation in Bacillus subtilis. Microbiol Sci. 1987 Aug;4(8):238–244. [PubMed] [Google Scholar]
- Moir A. Germination properties of a spore coat-defective mutant of Bacillus subtilis. J Bacteriol. 1981 Jun;146(3):1106–1116. doi: 10.1128/jb.146.3.1106-1116.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir A., Lafferty E., Smith D. A. Genetics analysis of spore germination mutants of Bacillus subtilis 168: the correlation of phenotype with map location. J Gen Microbiol. 1979 Mar;111(1):165–180. doi: 10.1099/00221287-111-1-165. [DOI] [PubMed] [Google Scholar]
- Piggot P. J., Coote J. G. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976 Dec;40(4):908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot P. J. Mapping of asporogenous mutations of Bacillus subtilis: a minimum estimate of the number of sporeulation operons. J Bacteriol. 1973 Jun;114(3):1241–1253. doi: 10.1128/jb.114.3.1241-1253.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Scott I. R., Ellar D. J. Study of calcium dipicolinate release during bacterial spore germination by using a new, sensitive assay for dipicolinate. J Bacteriol. 1978 Jul;135(1):133–137. doi: 10.1128/jb.135.1.133-137.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterlini J. M., Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969 Jun;113(1):29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S. M., Errington J., Mandelstam J. Use of a lacZ gene fusion to determine the dependence pattern of sporulation operon spoIIIC in spo mutants of Bacillus subtilis: a branched pathway of expression of sporulation operons. J Gen Microbiol. 1986 Nov;132(11):2995–3003. doi: 10.1099/00221287-132-11-2995. [DOI] [PubMed] [Google Scholar]
- Waites W. M., Kay D., Dawes I. W., Wood D. A., Warren S. C., Mandelstam J. Sporulation in Bacillus subtilis. Correlation of biochemical events with morphological changes in asporogenous mutants. Biochem J. 1970 Jul;118(4):667–676. doi: 10.1042/bj1180667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. B., Jr, Zahler S. A. Genetic studies of leucine biosynthesis in Bacillus subtilis. J Bacteriol. 1973 Nov;116(2):719–726. doi: 10.1128/jb.116.2.719-726.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



