Abstract
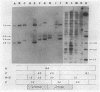
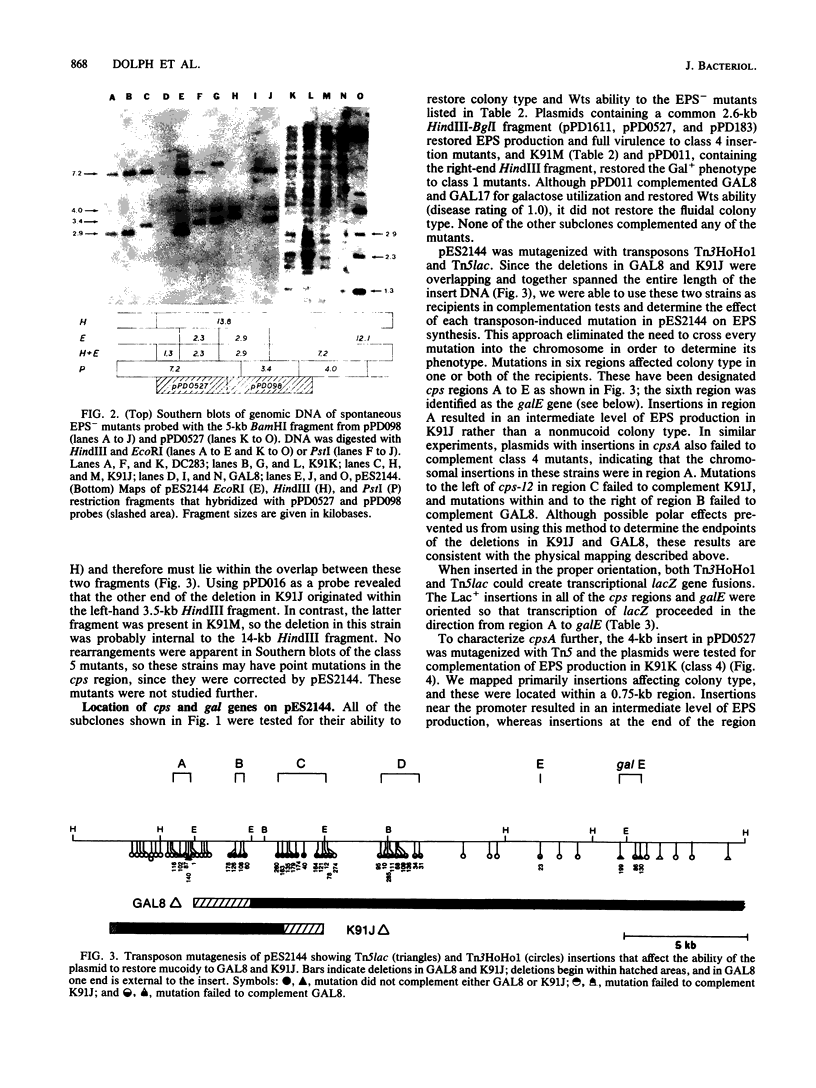
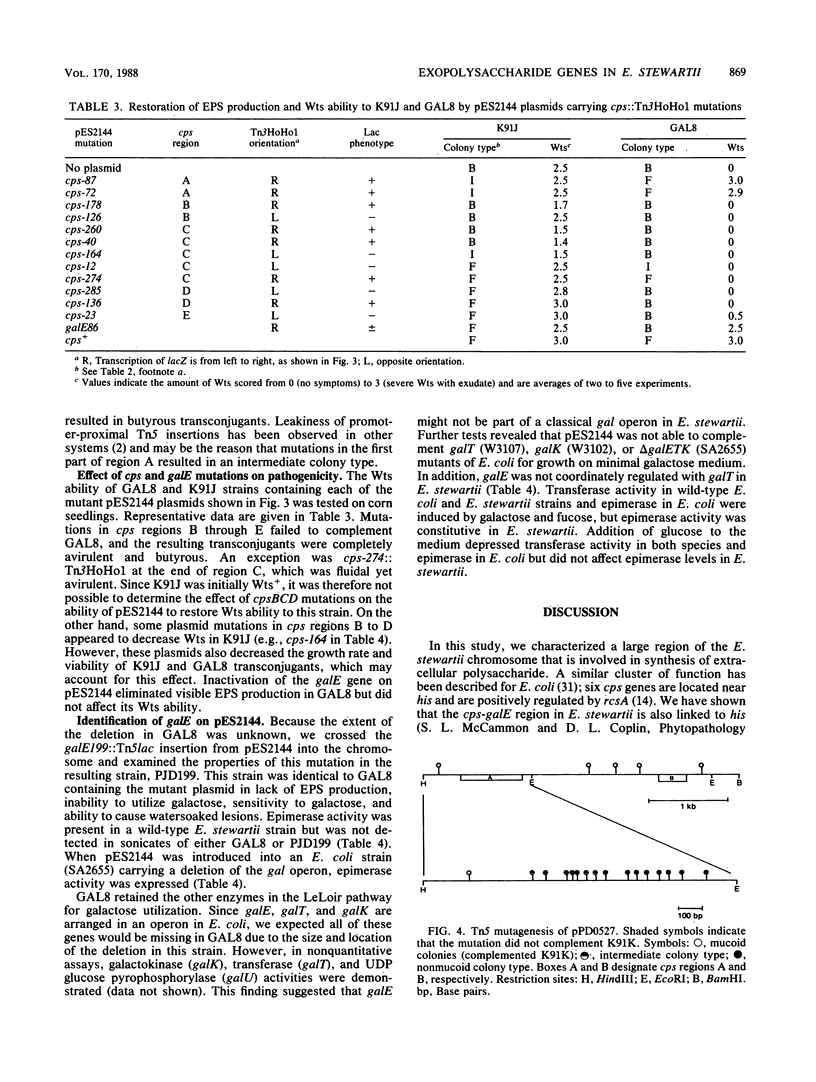
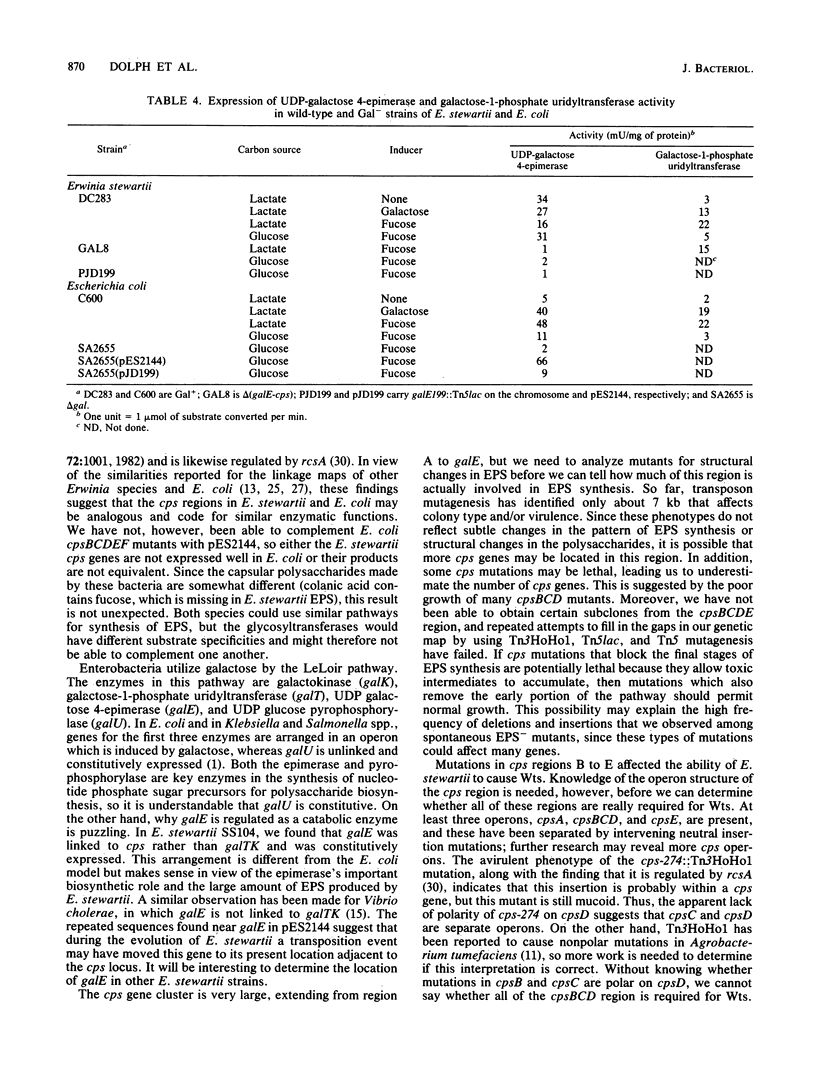
We have previously cloned the genes for synthesis of capsular polysaccharide (cps) and slime from Erwinia stewartii in cosmid pES2144. In this study, pES2144 was shown to complement 14 spontaneous cps mutants. These mutants were characterized by probing Southern blots of mutant genomic DNA with pES2144; insertions were detected in four mutants and deletions in six mutants. Genetic and physical maps of the pES2144 cps region were constructed by subcloning, restriction analysis, and transposon mutagenesis with Tn5, Tn5lac, and Tn3HoHo1. Mutations affecting the ability of pES2144 to restore mucoidy to cps deletion mutants were located in five regions, designated cpsA to cpsE. None of the cps mutants were able to cause systemic wilting of corn plants, and mutations in cps regions B to E further abolished the ability of the bacterium to cause watersoaked lesions on seedlings. The gene for uridine-5'-diphosphogalactose 4-epimerase (galE) was linked to the cps genes on pES2144. In E. stewartii, galE was constitutively expressed, whereas the genes for galactokinase (galK) and galactose-1-phosphate uridyltransferase (galT) were inducible and not linked to galE. Thus, galE does not appear to be part of the gal operon in this species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S. L., Shapiro J. A. The galactose operon of E. coli K-12. I. Structural and pleiotropic mutations of the operon. Genetics. 1969 Jun;62(2):231–247. doi: 10.1093/genetics/62.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Weiss A., Crossland L. Polarity of Tn5 insertion mutations in Escherichia coli. J Bacteriol. 1980 May;142(2):439–446. doi: 10.1128/jb.142.2.439-446.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw-Rouse J. J., Whatley M. H., Coplin D. L., Woods A., Sequeira L., Kelman A. Agglutination of Erwinia stewartii Strains with a Corn Agglutinin: Correlation with Extracellular Polysaccharide Production and Pathogenicity. Appl Environ Microbiol. 1981 Aug;42(2):344–350. doi: 10.1128/aem.42.2.344-350.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplin D. L., Frederick R. D., Majerczak D. R., Haas E. S. Molecular cloning of virulence genes from Erwinia stewartii. J Bacteriol. 1986 Nov;168(2):619–623. doi: 10.1128/jb.168.2.619-623.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplin D. L., Rowan R. G., Chisholm D. A., Whitmoyer R. E. Characterization of plasmids in Erwinia stewartii. Appl Environ Microbiol. 1981 Oct;42(4):599–604. doi: 10.1128/aem.42.4.599-604.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. J., Staneloni R. J., Rubin R. A., Nester E. W. Identification and genetic analysis of an Agrobacterium tumefaciens chromosomal virulence region. J Bacteriol. 1985 Mar;161(3):850–860. doi: 10.1128/jb.161.3.850-860.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietta A., Grandi G., Malcovati M., Valentini G., Sgaramella V., Siccardi A. G. R-factor-mediated suppression of the galactose-sensitive phenotype of Escherichia coli K-12 galE Mutants. Plasmid. 1981 Jul;6(1):78–85. doi: 10.1016/0147-619x(81)90055-x. [DOI] [PubMed] [Google Scholar]
- Forbes K. J., Pérombelon M. C. Chromosomal mapping in Erwinia carotovora subsp. carotovora with the IncP plasmid R68::Mu. J Bacteriol. 1985 Dec;164(3):1110–1116. doi: 10.1128/jb.164.3.1110-1116.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S., Trisler P., Torres-Cabassa A. Regulation of capsular polysaccharide synthesis in Escherichia coli K-12: characterization of three regulatory genes. J Bacteriol. 1985 Jun;162(3):1111–1119. doi: 10.1128/jb.162.3.1111-1119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanoff S. S., Riker A. J., Dettwiler H. A. STUDIES ON CULTURAL CHARACTERISTICS, PHYSIOLOGY AND PATHOGENICITY OF STRAIN TYPES OF PHYTOMONAS STEWARTI. J Bacteriol. 1938 Mar;35(3):235–253. doi: 10.1128/jb.35.3.235-253.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- Kroos L., Kaiser D. Construction of Tn5 lac, a transposon that fuses lacZ expression to exogenous promoters, and its introduction into Myxococcus xanthus. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5816–5820. doi: 10.1073/pnas.81.18.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh J. A., Signer E. R., Walker G. C. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno F., Rodicio R., Herrero P. A new colorimetric assay for UDP-glucose 4-epimerase activity. Cell Mol Biol Incl Cyto Enzymol. 1981;27(6):589–592. [PubMed] [Google Scholar]
- Pugashetti B. K., Chatterjee A. K., Starr M. P. Isolation and characterization of Hfr strains of Erwinia amylovora. Can J Microbiol. 1978 Apr;24(4):448–454. doi: 10.1139/m78-074. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- Schoonejans E., Toussaint A. Utilization of plasmid pULB113 (RP4::mini-Mu) to construct a linkage map of Erwinia carotovora subsp. chrysanthemi. J Bacteriol. 1983 Jun;154(3):1489–1492. doi: 10.1128/jb.154.3.1489-1492.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Stachel S. E., An G., Flores C., Nester E. W. A Tn3 lacZ transposon for the random generation of beta-galactosidase gene fusions: application to the analysis of gene expression in Agrobacterium. EMBO J. 1985 Apr;4(4):891–898. doi: 10.1002/j.1460-2075.1985.tb03715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Cabassa A., Gottesman S., Frederick R. D., Dolph P. J., Coplin D. L. Control of extracellular polysaccharide synthesis in Erwinia stewartii and Escherichia coli K-12: a common regulatory function. J Bacteriol. 1987 Oct;169(10):4525–4531. doi: 10.1128/jb.169.10.4525-4531.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trisler P., Gottesman S. lon transcriptional regulation of genes necessary for capsular polysaccharide synthesis in Escherichia coli K-12. J Bacteriol. 1984 Oct;160(1):184–191. doi: 10.1128/jb.160.1.184-191.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]