Abstract
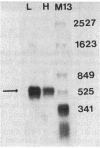
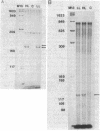
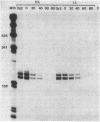
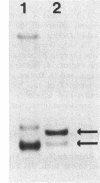
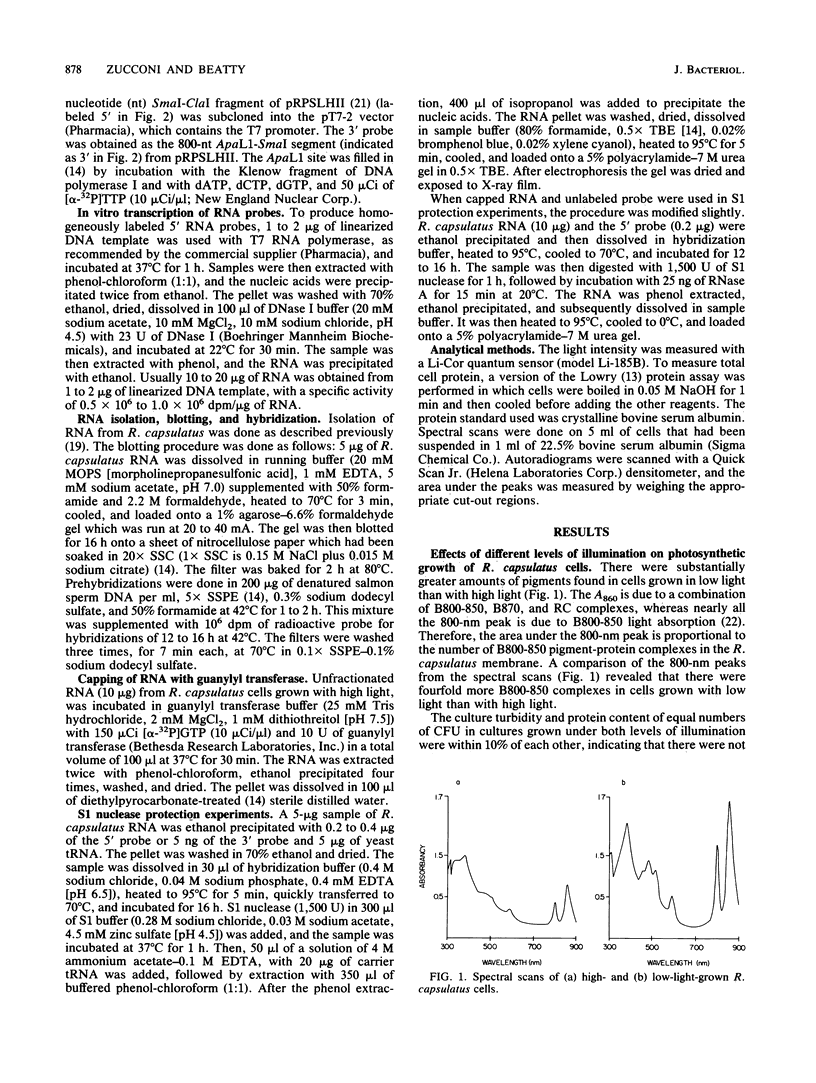
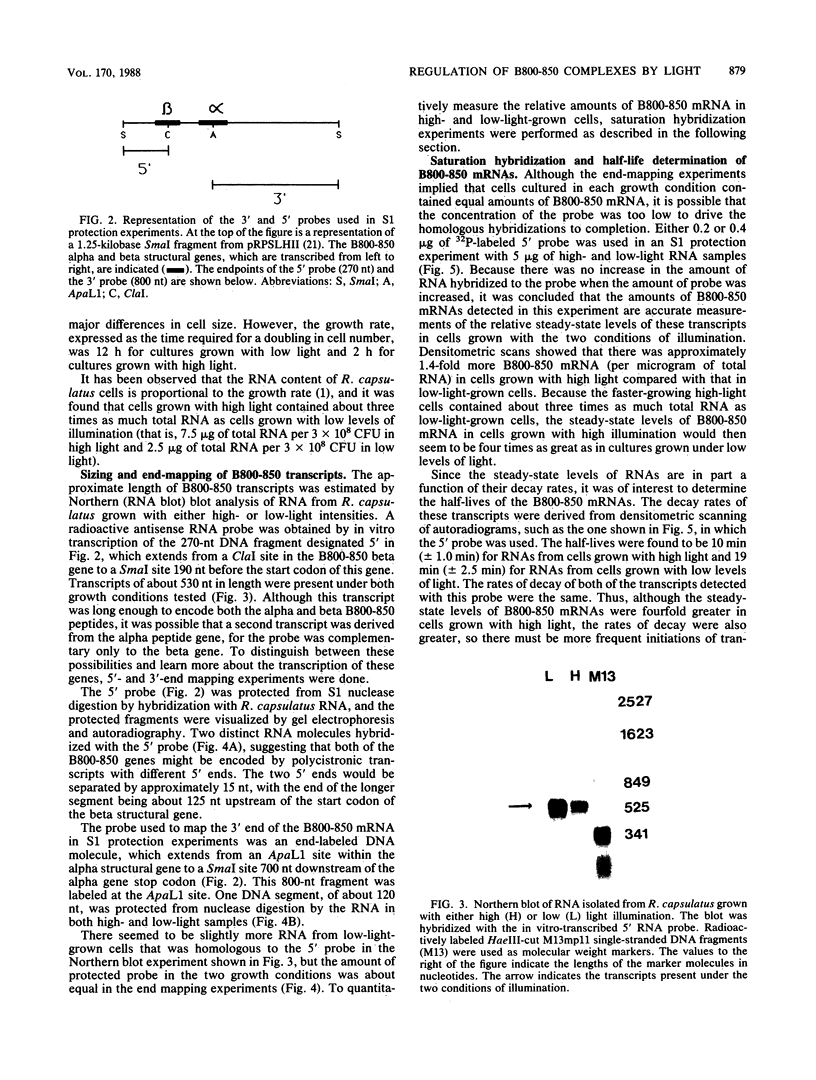
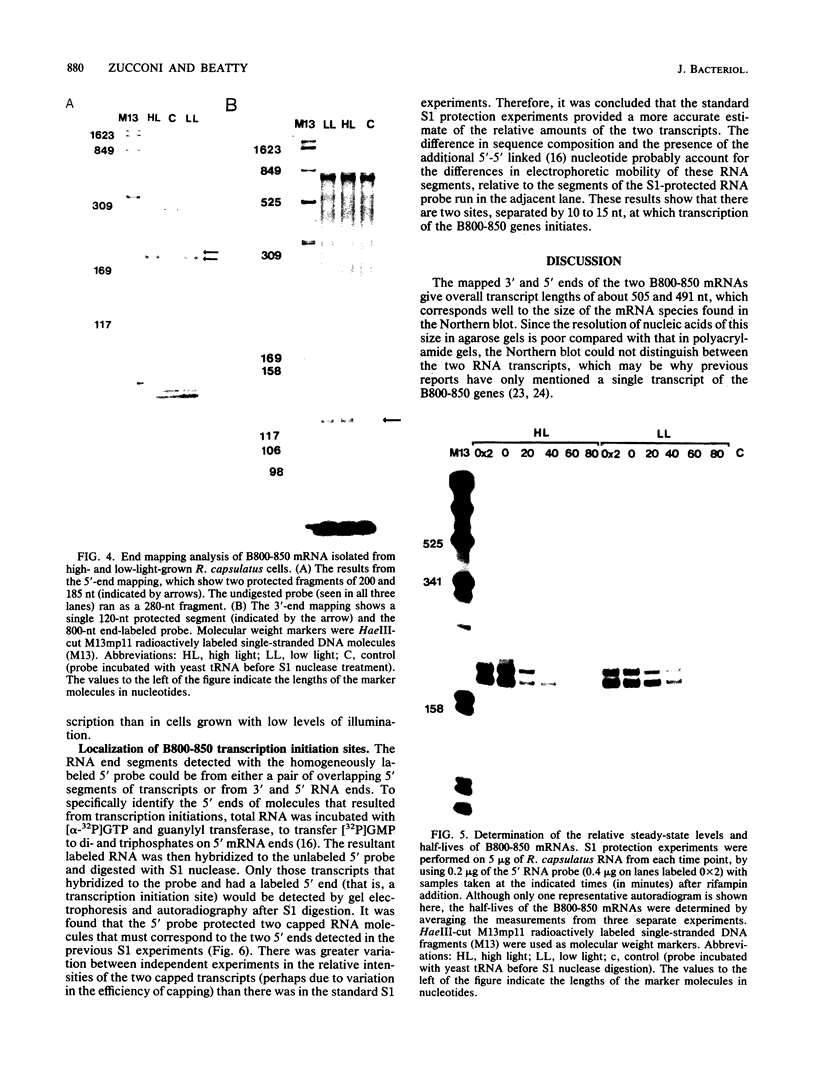
Photosynthetic organisms exhibit a variety of responses to changes in light intensity, including differential biosynthesis of chlorophyll-protein complexes. Cultures of Rhodobacter capsulatus grown anaerobically with a low intensity of light (2 W/m2) contained about four times as much B800-850 light-harvesting complex as cells grown under high light intensity (140 W/m2). The mRNA transcripts encoding B800-850 beta and alpha peptides were analyzed by Northern blot (RNA blot), S1 nuclease protection, and capping with guanylyl transferase. It was found that the steady-state levels of B800-850 mRNAs in high-light-grown cultures were about four times as great as in cells grown under low light intensity. Therefore, the lesser amounts of mature B800-850 peptide gene products found in cells grown with high light intensity are the result of a posttranscriptional regulatory process. It was also found that there are two polycistronic messages encoding the B800-850 peptides. These messages share a common 3' terminus but differ in their 5'-end segments as a result of transcription initiation at two discrete sites. Moreover, the half-lives of B800-850 mRNAs were about 10 min in cells grown with high light and approximately 19 min in cultures grown with low light. It is concluded that there must be more frequent initiations of transcription of B800-850 genes in cells grown with high light than in those grown with low light, and that the relative amounts of B800-850 complexes under these conditions are controlled by a translational or posttranslational mechanism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiking H., Sojka G. Response of Rhodopseudomonas capsulata to illumination and growth rate in a light-limited continuous culture. J Bacteriol. 1979 Aug;139(2):530–536. doi: 10.1128/jb.139.2.530-536.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belasco J. G., Beatty J. T., Adams C. W., von Gabain A., Cohen S. N. Differential expression of photosynthesis genes in R. capsulata results from segmental differences in stability within the polycistronic rxcA transcript. Cell. 1985 Jan;40(1):171–181. doi: 10.1016/0092-8674(85)90320-4. [DOI] [PubMed] [Google Scholar]
- Biel A. J. Control of bacteriochlorophyll accumulation by light in Rhodobacter capsulatus. J Bacteriol. 1986 Nov;168(2):655–659. doi: 10.1128/jb.168.2.655-659.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. G., Davidson E., Marrs B. L. Variation of levels of mRNA coding for antenna and reaction center polypeptides in Rhodopseudomonas capsulata in response to changes in oxygen concentration. J Bacteriol. 1984 Mar;157(3):945–948. doi: 10.1128/jb.157.3.945-948.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierstein R. Synthesis of pigment-binding protein in toluene-treated Rhodopseudomonas capsulata and in cell-free systems. Eur J Biochem. 1984 Feb 1;138(3):509–518. doi: 10.1111/j.1432-1033.1984.tb07945.x. [DOI] [PubMed] [Google Scholar]
- Drews G., Oelze J. Organization and differentiation of membranes of phototrophic bacteria. Adv Microb Physiol. 1981;22:1–92. doi: 10.1016/s0065-2911(08)60325-2. [DOI] [PubMed] [Google Scholar]
- Johnson J. A., Wong W. K., Beatty J. T. Expression of cellulase genes in Rhodobacter capsulatus by use of plasmid expression vectors. J Bacteriol. 1986 Aug;167(2):604–610. doi: 10.1128/jb.167.2.604-610.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug G., Kaufmann N., Drews G. Gene expression of pigment-binding proteins of the bacterial photosynthetic apparatus: Transcription and assembly in the membrane of Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6485–6489. doi: 10.1073/pnas.82.19.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lien S., Gest H., San Pietro A. Regulation of chlorophyll synthesis in photosynthetic bacteria. J Bioenerg. 1973;4(4):423–434. doi: 10.1007/BF01648969. [DOI] [PubMed] [Google Scholar]
- Marrs B. Genetic recombination in Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1974 Mar;71(3):971–973. doi: 10.1073/pnas.71.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. 5' end labeling of RNA with capping and methylating enzymes. Gene Amplif Anal. 1981;2:253–266. [PubMed] [Google Scholar]
- Schumacher A., Drews G. Effects of light intensity on membrane differentiation in Rhodopseudomonas capsulata. Biochim Biophys Acta. 1979 Sep 11;547(3):417–428. doi: 10.1016/0005-2728(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Shiozawa J. A., Cuendet P. A., Drews G., Zuber H. Isolation and characterization of the polypeptide components from light-harvesting pigment-protein complex B800--850 of Rhodopseudomonas capsulata. Eur J Biochem. 1980 Oct;111(2):455–460. doi: 10.1111/j.1432-1033.1980.tb04960.x. [DOI] [PubMed] [Google Scholar]
- Weaver P. F., Wall J. D., Gest H. Characterization of Rhodopseudomonas capsulata. Arch Microbiol. 1975 Nov 7;105(3):207–216. doi: 10.1007/BF00447139. [DOI] [PubMed] [Google Scholar]
- Youvan D. C., Ismail S., Bylina E. J. Chromosomal deletion and plasmid complementation of the photosynthetic reaction center and light-harvesting genes from Rhodopseudomonas capsulata. Gene. 1985;38(1-3):19–30. doi: 10.1016/0378-1119(85)90199-4. [DOI] [PubMed] [Google Scholar]
- Youvan D. C., Ismail S. Light-harvesting II (B800-B850 complex) structural genes from Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1985 Jan;82(1):58–62. doi: 10.1073/pnas.82.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. S., Cook D. N., Leach F., Armstrong G. A., Alberti M., Hearst J. E. Oxygen-regulated mRNAs for light-harvesting and reaction center complexes and for bacteriochlorophyll and carotenoid biosynthesis in Rhodobacter capsulatus during the shift from anaerobic to aerobic growth. J Bacteriol. 1986 Dec;168(3):1180–1188. doi: 10.1128/jb.168.3.1180-1188.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. S., Hearst J. E. Regulation of expression of genes for light-harvesting antenna proteins LH-I and LH-II; reaction center polypeptides RC-L, RC-M, and RC-H; and enzymes of bacteriochlorophyll and carotenoid biosynthesis in Rhodobacter capsulatus by light and oxygen. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7613–7617. doi: 10.1073/pnas.83.20.7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gabain A., Belasco J. G., Schottel J. L., Chang A. C., Cohen S. N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci U S A. 1983 Feb;80(3):653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]