Abstract
In Bacillus subtilis, the formation of glutaminyl-tRNA is accomplished by first charging tRNA(Gln) with glutamate, which is then amidated. Glutamine was preferred over asparagine and ammonia as the amide donor in vitro. There is a functional analogy of this reaction to that catalyzed by glutamine synthetase. Homogeneous glutamine synthetase, from either B. subtilis or Escherichia coli, catalyzed the amidotransferase reaction but only about 3 to 5% as well as a partially purified preparation from B. subtilis. Several classes of glutamine synthetase mutants of B. subtilis, however, were unaltered in the amidotransferase reaction. In addition, there was no inhibition by inhibitors of either glutamine synthetase or other amidotransferases. A unique, rather labile activity seems to be required for this reaction.
Full text
PDF
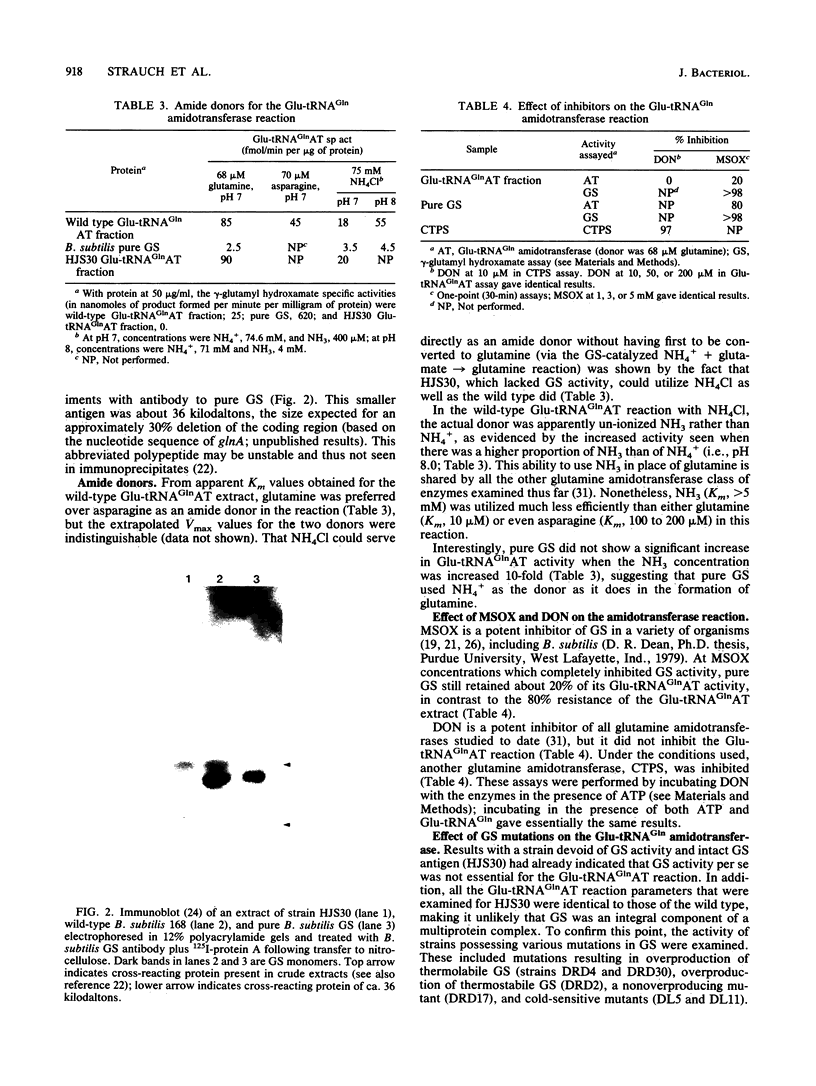


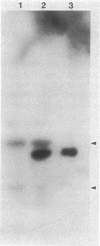
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M. CTP synthetase from Escherichia coli: an improved purification procedure and characterization of hysteretic and enzyme concentration effects on kinetic properties. Biochemistry. 1983 Jun 21;22(13):3285–3292. doi: 10.1021/bi00282a038. [DOI] [PubMed] [Google Scholar]
- Bozouklian H., Elmerich C. Nucleotide sequence of the Azospirillum brasilense Sp7 glutamine synthetase structural gene. Biochimie. 1986 Oct-Nov;68(10-11):1181–1187. doi: 10.1016/s0300-9084(86)80062-1. [DOI] [PubMed] [Google Scholar]
- Buchanan J. M. The amidotransferases. Adv Enzymol Relat Areas Mol Biol. 1973;39:91–183. doi: 10.1002/9780470122846.ch2. [DOI] [PubMed] [Google Scholar]
- Dean D. R., Aronson A. I. Selection of Bacillus subtilis mutants impaired in ammonia assimilation. J Bacteriol. 1980 Feb;141(2):985–988. doi: 10.1128/jb.141.2.985-988.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. R., Hoch J. A., Aronson A. I. Alteration of the Bacillus subtilis glutamine synthetase results in overproduction of the enzyme. J Bacteriol. 1977 Sep;131(3):981–987. doi: 10.1128/jb.131.3.981-987.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Ginsburg A., Yeh J., Shelton E., Stadtman E. R. Bacillus subtilis glutamine synthetase. Purification and physical characterization. J Biol Chem. 1970 Oct 25;245(20):5195–5205. [PubMed] [Google Scholar]
- Fisher S. H., Sonenshein A. L. Bacillus subtilis glutamine synthetase mutants pleiotropically altered in glucose catabolite repression. J Bacteriol. 1984 Feb;157(2):612–621. doi: 10.1128/jb.157.2.612-621.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S. H., Sonenshein A. L. Glutamine-requiring mutants of Bacillus subtilis. Biochem Biophys Res Commun. 1977 Dec 7;79(3):987–995. doi: 10.1016/0006-291x(77)91207-4. [DOI] [PubMed] [Google Scholar]
- Janson C. A., Kayne P. S., Almassy R. J., Grunstein M., Eisenberg D. Sequence of glutamine synthetase from Salmonella typhimurium and implications for the protein structure. Gene. 1986;46(2-3):297–300. doi: 10.1016/0378-1119(86)90415-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lapointe J., Duplain L., Proulx M. A single glutamyl-tRNA synthetase aminoacylates tRNAGlu and tRNAGln in Bacillus subtilis and efficiently misacylates Escherichia coli tRNAGln1 in vitro. J Bacteriol. 1986 Jan;165(1):88–93. doi: 10.1128/jb.165.1.88-93.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitzki A., Koshland D. E., Jr Cytidine triphosphate synthetase. Covalent intermediates and mechanisms of action. Biochemistry. 1971 Aug 31;10(18):3365–3371. doi: 10.1021/bi00794a008. [DOI] [PubMed] [Google Scholar]
- Martin N. C., Rabinowitz M., Fukuhara H. Yeast mitochondrial DNA specifies tRNA for 19 amino acids. Deletion mapping of the tRNA genes. Biochemistry. 1977 Oct 18;16(21):4672–4677. doi: 10.1021/bi00640a022. [DOI] [PubMed] [Google Scholar]
- Martin N., Rabinowitz M., Fukuhara H. Isoaccepting mitochondrial glutamyl-tRNA species transcribed from different regions of the mitochondrial genome of Saccharomyces cerevisiae. J Mol Biol. 1976 Mar 5;101(3):285–296. doi: 10.1016/0022-2836(76)90148-0. [DOI] [PubMed] [Google Scholar]
- PACE J., McDERMOTT E. E. Methionine sulphoximine and some enzyme systems in volving glutamine. Nature. 1952 Mar 8;169(4297):415–416. doi: 10.1038/169415a0. [DOI] [PubMed] [Google Scholar]
- Proulx M., Duplain L., Lacoste L., Yaguchi M., Lapointe J. The monomeric glutamyl-tRNA synthetase from Bacillus subtilis 168 and its regulatory factor. Their purification, characterization, and the study of their interaction. J Biol Chem. 1983 Jan 25;258(2):753–759. [PubMed] [Google Scholar]
- Reysset G. New class of Bacillus subtilis glutamine-requiring mutants. J Bacteriol. 1981 Nov;148(2):653–658. doi: 10.1128/jb.148.2.653-658.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronzio R. A., Rowe W. B., Meister A. Studies on the mechanism of inhibition of glutamine synthetase by methionine sulfoximine. Biochemistry. 1969 Mar;8(3):1066–1075. doi: 10.1021/bi00831a038. [DOI] [PubMed] [Google Scholar]
- Schreier H. J., Sonenshein A. L. Altered regulation of the glnA gene in glutamine synthetase mutants of Bacillus subtilis. J Bacteriol. 1986 Jul;167(1):35–43. doi: 10.1128/jb.167.1.35-43.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimer-Veale K., Brenchley J. E. Characterization of Salmonella typhimurium strains sensitive and resistant to methionine sulfoximine. J Bacteriol. 1974 Sep;119(3):848–856. doi: 10.1128/jb.119.3.848-856.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vold B. S. Preparation of tRNA's and aminoacyl-tRNA synthetases from Bacillus subtilis cells at various stages of growth and spores. Methods Enzymol. 1974;29:502–510. doi: 10.1016/0076-6879(74)29045-1. [DOI] [PubMed] [Google Scholar]
- Weisbrod R. E., Meister A. Studies on glutamine synthetase from Escherichia coli. Formation of pyrrolidone carboxylate and inhibition by methionine sulfoximine. J Biol Chem. 1973 Jun 10;248(11):3997–4002. [PubMed] [Google Scholar]
- Weng M., Makaroff C. A., Zalkin H. Nucleotide sequence of Escherichia coli pyrG encoding CTP synthetase. J Biol Chem. 1986 Apr 25;261(12):5568–5574. [PubMed] [Google Scholar]
- Wilcox M. Gamma-glutamyl phosphate attached to glutamine-specific tRNA. A precursor of glutaminyl-tRNA in Bacillus subtilis. Eur J Biochem. 1969 Dec;11(3):405–412. doi: 10.1111/j.1432-1033.1969.tb00788.x. [DOI] [PubMed] [Google Scholar]
- Wilcox M. Gamma-phosphoryl ester of glu-tRNA-GLN as an intermediate in Bacillus subtilis glutaminyl-tRNA synthesis. Cold Spring Harb Symp Quant Biol. 1969;34:521–528. doi: 10.1101/sqb.1969.034.01.059. [DOI] [PubMed] [Google Scholar]
- Wilcox M., Nirenberg M. Transfer RNA as a cofactor coupling amino acid synthesis with that of protein. Proc Natl Acad Sci U S A. 1968 Sep;61(1):229–236. doi: 10.1073/pnas.61.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalkin H. Glutamine amidotransferases. Methods Enzymol. 1985;113:263–264. doi: 10.1016/s0076-6879(85)13035-1. [DOI] [PubMed] [Google Scholar]