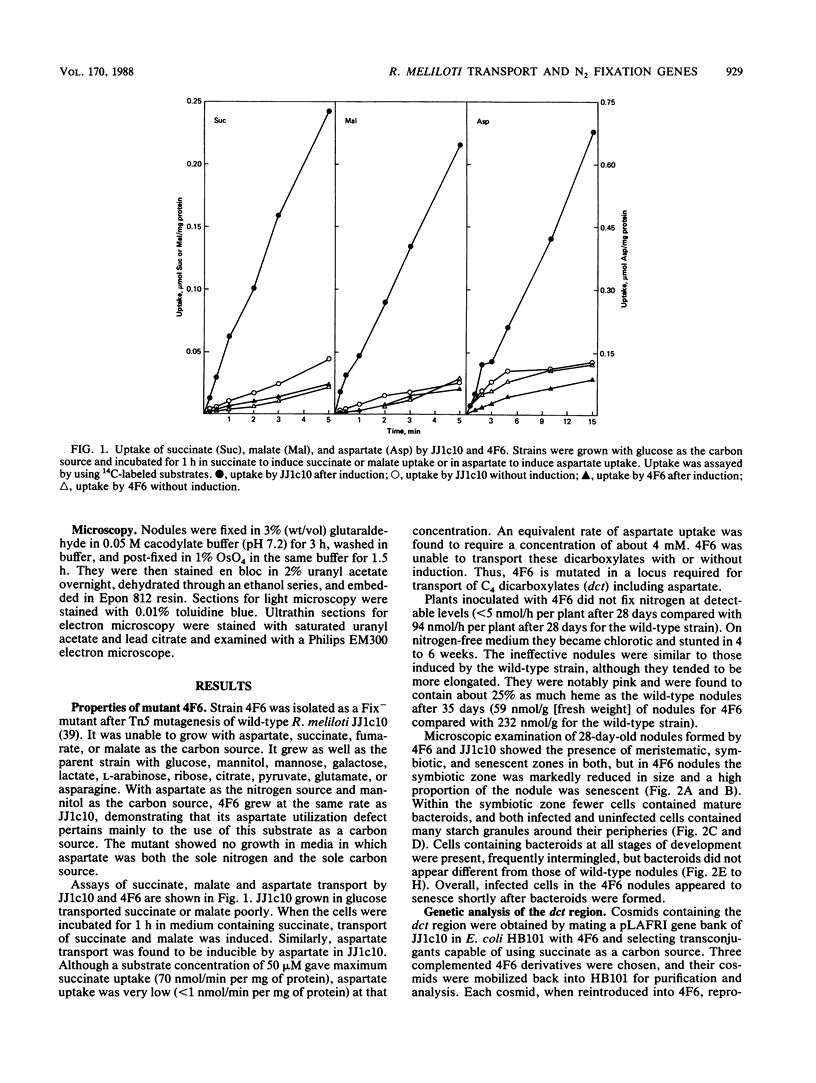
Abstract
A mutant of Rhizobium meliloti unable to transport C4 dicarboxylates (dct) was isolated after Tn5 mutagenesis. The mutant, 4F6, could not grow on aspartate or the tricarboxylic acid cycle intermediates succinate, fumarate, or malate. It produced symbiotically ineffective nodules on Medicago sativa in which bacteroids appeared normal, but the symbiotic zone was reduced and the plant cells contained numerous starch granules at their peripheries. Cosmids containing the dct region were obtained by selecting those which restored the ability of 4F6 to grow on succinate. The Tn5 insertion in 4F6 was found to be within a 5.9-kilobase (kb) EcoRI fragment common to the complementing cosmids. Site-specific Tn5-mutagenesis revealed dct genes in a segment of DNA about 4 kb in size extending from within the 5.9-kb EcoRI fragment into an adjacent 2.9-kb EcoRI fragment. The 4F6 mutation was found to be in a complementation group in which mutations yielded a Fix- phenotype, whereas other dct mutations in the region resulted in mutants which produced effective nodules in most, although not all, plant tests (partially Fix-). The dct region was found to be located on a megaplasmid known to carry genes required for exopolysaccharide production.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergersen J. F., Turner G. L. Nitrogen fixation by the bacteroid fraction of breis of soybean root nodules. Biochim Biophys Acta. 1967 Aug 29;141(3):507–515. doi: 10.1016/0304-4165(67)90179-1. [DOI] [PubMed] [Google Scholar]
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Buikema W. J., Long S. R., Brown S. E., van den Bos R. C., Earl C., Ausubel F. M. Physical and genetic characterization of Rhizobium meliloti symbiotic mutants. J Mol Appl Genet. 1983;2(3):249–260. [PubMed] [Google Scholar]
- Bánfalvi Z., Sakanyan V., Koncz C., Kiss A., Dusha I., Kondorosi A. Location of nodulation and nitrogen fixation genes on a high molecular weight plasmid of R. meliloti. Mol Gen Genet. 1981;184(2):318–325. doi: 10.1007/BF00272925. [DOI] [PubMed] [Google Scholar]
- Ditta G., Schmidhauser T., Yakobson E., Lu P., Liang X. W., Finlay D. R., Guiney D., Helinski D. R. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid. 1985 Mar;13(2):149–153. doi: 10.1016/0147-619x(85)90068-x. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan M. J., Fraenkel D. G. alpha-Ketoglutarate dehydrogenase mutant of Rhizobium meliloti. J Bacteriol. 1979 Jan;137(1):415–419. doi: 10.1128/jb.137.1.415-419.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan T. M., Hirsch A. M., Leigh J. A., Johansen E., Kuldau G. A., Deegan S., Walker G. C., Signer E. R. Symbiotic mutants of Rhizobium meliloti that uncouple plant from bacterial differentiation. Cell. 1985 Apr;40(4):869–877. doi: 10.1016/0092-8674(85)90346-0. [DOI] [PubMed] [Google Scholar]
- Finan T. M., Kunkel B., De Vos G. F., Signer E. R. Second symbiotic megaplasmid in Rhizobium meliloti carrying exopolysaccharide and thiamine synthesis genes. J Bacteriol. 1986 Jul;167(1):66–72. doi: 10.1128/jb.167.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan T. M., Wood J. M., Jordan D. C. Symbiotic properties of C4-dicarboxylic acid transport mutants of Rhizobium leguminosarum. J Bacteriol. 1983 Jun;154(3):1403–1413. doi: 10.1128/jb.154.3.1403-1413.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrai T., Vincze E., Bánfalvi Z., Kiss G. B., Randhawa G. S., Kondorosi A. Localization of symbiotic mutations in Rhizobium meliloti. J Bacteriol. 1983 Feb;153(2):635–643. doi: 10.1128/jb.153.2.635-643.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A. M., Long S. R., Brown S. E., Buikema W. J., Ausubel F. M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982 Jun;18(3):289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- Gardiol A., Arias A., Cerveñansky C., Martínez-Drets G. Succinate dehydrogenase mutant of Rhizobium meliloti. J Bacteriol. 1982 Sep;151(3):1621–1623. doi: 10.1128/jb.151.3.1621-1623.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot M. L., Chelm B. K. Bacterial delta-aminolevulinic acid synthase activity is not essential for leghemoglobin formation in the soybean/Bradyrhizobium japonicum symbiosis. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1837–1841. doi: 10.1073/pnas.83.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer V. N., Klee H. J., Nester E. W. Units of genetic expression in the virulence region of a plant tumor-inducing plasmid of Agrobacterium tumefaciens. Mol Gen Genet. 1982;188(3):418–424. doi: 10.1007/BF00330043. [DOI] [PubMed] [Google Scholar]
- Kay W. W., Kornberg H. L. Genetic control of the uptake of C(4)-dicarboxylic acids by Escherichia coli. FEBS Lett. 1969 Apr;3(2):93–96. doi: 10.1016/0014-5793(69)80105-5. [DOI] [PubMed] [Google Scholar]
- Leigh J. A., Signer E. R., Walker G. C. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade H. M., Long S. R., Ruvkun G. B., Brown S. E., Ausubel F. M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982 Jan;149(1):114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ronson C. W., Astwood P. M., Downie J. A. Molecular cloning and genetic organization of C4-dicarboxylate transport genes from Rhizobium leguminosarum. J Bacteriol. 1984 Dec;160(3):903–909. doi: 10.1128/jb.160.3.903-909.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronson C. W., Lyttleton P., Robertson J. G. C(4)-dicarboxylate transport mutants of Rhizobium trifolii form ineffective nodules on Trifolium repens. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4284–4288. doi: 10.1073/pnas.78.7.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg C., Boistard P., Dénarié J., Casse-Delbart F. Genes controlling early and late functions in symbiosis are located on a megaplasmid in Rhizobium meliloti. Mol Gen Genet. 1981;184(2):326–333. doi: 10.1007/BF00272926. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. B., Ausubel F. M. A general method for site-directed mutagenesis in prokaryotes. Nature. 1981 Jan 1;289(5793):85–88. doi: 10.1038/289085a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stowers M. D. Carbon metabolism in Rhizobium species. Annu Rev Microbiol. 1985;39:89–108. doi: 10.1146/annurev.mi.39.100185.000513. [DOI] [PubMed] [Google Scholar]




