Abstract
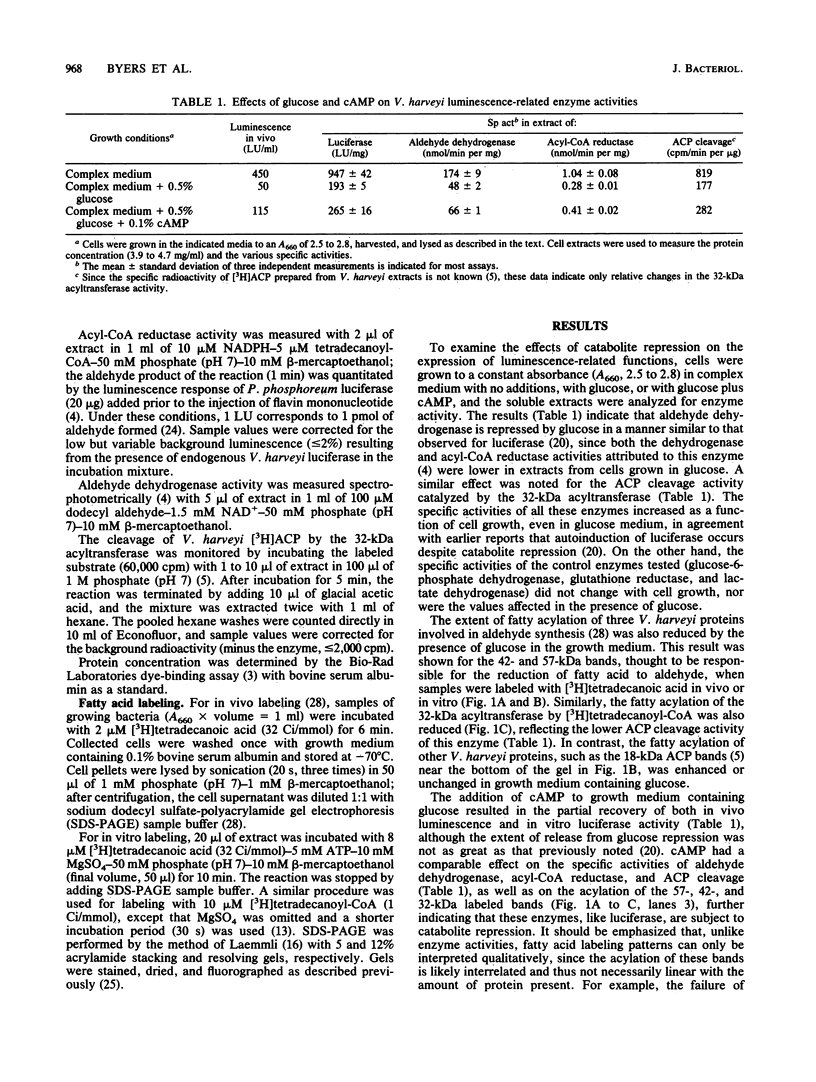
The effects of catabolite repression and nutrient abundance on the activities of Vibrio harveyi enzymes known to be related to aldehyde metabolism were investigated. The growth of cells in complex medium containing glucose, which decreases in vivo luminescence and luciferase synthesis, also resulted in decreases in the specific activities of V. harveyi aldehyde dehydrogenase and acyl carrier protein acyltransferase as well as in the degree of fatty acylation of three bioluminescence-specific polypeptides (32, 42, and 57 kilodaltons), as monitored by sodium dodecyl sulfatepolyacrylamide gel electrophoresis. This repression was partially alleviated in glucose medium containing cyclic AMP. The acylation of the above-mentioned proteins, in addition to light emission and luciferase and acyltransferase activities, was also repressed when cells were grown in minimal medium, with partial recovery of these functions upon the addition of arginine. In contrast, aldehyde dehydrogenase activity was increased in minimal medium. These results suggest that the 42-, 57-, and 32-kilodalton proteins, which are responsible for the supply and reduction of fatty acids to form aldehydes for the luciferase reaction, are regulated in the same way as luciferase under the above-described conditions. However, aldehyde dehydrogenase, whose role in V. harveyi aldehyde metabolism is not yet known, is regulated in a different way with respect to nutrient composition.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botsford J. L. Cyclic nucleotides in procaryotes. Microbiol Rev. 1981 Dec;45(4):620–642. doi: 10.1128/mr.45.4.620-642.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan M., Graham A. F., Meighen E. A. Functional identification of the fatty acid reductase components encoded in the luminescence operon of Vibrio fischeri. J Bacteriol. 1985 Sep;163(3):1186–1190. doi: 10.1128/jb.163.3.1186-1190.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Byers D. M., Meighen E. A. Acyl-acyl carrier protein as a source of fatty acids for bacterial bioluminescence. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6085–6089. doi: 10.1073/pnas.82.18.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers D., Meighen E. Vibrio harveyi aldehyde dehydrogenase. Partial reversal of aldehyde oxidation and its possible role in the reduction of fatty acids for the bioluminescence reaction. J Biol Chem. 1984 Jun 10;259(11):7109–7114. [PubMed] [Google Scholar]
- Carey L. M., Rodriguez A., Meighen E. Generation of fatty acids by an acyl esterase in the bioluminescent system of Photobacterium phosphoreum. J Biol Chem. 1984 Aug 25;259(16):10216–10221. [PubMed] [Google Scholar]
- Coffey J. J. Inducible synthesis of bacterial luciferase: specificity and kinetics of induction. J Bacteriol. 1967 Nov;94(5):1638–1647. doi: 10.1128/jb.94.5.1638-1647.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap P. V., Greenberg E. P. Control of Vibrio fischeri luminescence gene expression in Escherichia coli by cyclic AMP and cyclic AMP receptor protein. J Bacteriol. 1985 Oct;164(1):45–50. doi: 10.1128/jb.164.1.45-50.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard A., Burlingame A. L., Eberhard C., Kenyon G. L., Nealson K. H., Oppenheimer N. J. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981 Apr 28;20(9):2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- Eberhard A. Inhibition and activation of bacterial luciferase synthesis. J Bacteriol. 1972 Mar;109(3):1101–1105. doi: 10.1128/jb.109.3.1101-1105.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebrecht J., Nealson K., Silverman M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983 Mar;32(3):773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- Engebrecht J., Silverman M. Identification of genes and gene products necessary for bacterial bioluminescence. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4154–4158. doi: 10.1073/pnas.81.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus-Miguel A., Meighen E. A., Nicoli M. Z., Nealson K. H., Hastings J. W. Purification and properties of bacterial luciferases. J Biol Chem. 1972 Jan 25;247(2):398–404. [PubMed] [Google Scholar]
- Hastings J. W., Nealson K. H. Bacterial bioluminescence. Annu Rev Microbiol. 1977;31:549–595. doi: 10.1146/annurev.mi.31.100177.003001. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Makemson J. C., Hastings J. W. Poising of the arginine pool and control of bioluminescence in Beneckea harveyi. J Bacteriol. 1979 Nov;140(2):532–542. doi: 10.1128/jb.140.2.532-542.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meighen E. A., Bogacki I. G., Bognar A., Michaliszyn G. A. Induction of fatty aldehyde dehydrogenase activity during the development of bioluminescence in Beneckea harveyi. Biochem Biophys Res Commun. 1976 Mar 22;69(2):423–430. doi: 10.1016/0006-291x(76)90539-8. [DOI] [PubMed] [Google Scholar]
- Miyamoto C. M., Graham A. D., Boylan M., Evans J. F., Hasel K. W., Meighen E. A., Graham A. F. Polycistronic mRNAs code for polypeptides of the Vibrio harveyi luminescence system. J Bacteriol. 1985 Mar;161(3):995–1001. doi: 10.1128/jb.161.3.995-1001.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealson K. H., Eberhard A., Hastings J. W. Catabolite repression of bacterial bioluminescence: functional implications. Proc Natl Acad Sci U S A. 1972 May;69(5):1073–1076. doi: 10.1073/pnas.69.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealson K. H., Platt T., Hastings J. W. Cellular control of the synthesis and activity of the bacterial luminescent system. J Bacteriol. 1970 Oct;104(1):313–322. doi: 10.1128/jb.104.1.313-322.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve C. A., Baldwin T. O. Luciferase inactivation in the luminous marine bacterium Vibrio harveyi. J Bacteriol. 1981 Jun;146(3):1038–1045. doi: 10.1128/jb.146.3.1038-1045.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riendeau D., Meighen E. Evidence for a fatty acid reductase catalyzing the synthesis of aldehydes for the bacterial bioluminescent reaction. Resolution from luciferase and dependence on fatty acids. J Biol Chem. 1979 Aug 25;254(16):7488–7490. [PubMed] [Google Scholar]
- Rodriguez A., Riendeau D., Meighen E. Purification of the acyl coenzyme A reductase component from a complex responsible for the reduction of fatty acids in bioluminescent bacteria. Properties and acyltransferase activity. J Biol Chem. 1983 Apr 25;258(8):5233–5237. [PubMed] [Google Scholar]
- Ulitzur S., Hastings J. W. Control of aldehyde synthesis in the luminous bacterium Beneckea harveyi. J Bacteriol. 1979 Feb;137(2):854–859. doi: 10.1128/jb.137.2.854-859.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall L. A., Byers D. M., Meighen E. A. In vivo and in vitro acylation of polypeptides in Vibrio harveyi: identification of proteins involved in aldehyde production for bioluminescence. J Bacteriol. 1984 Aug;159(2):720–724. doi: 10.1128/jb.159.2.720-724.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall L., Rodriquez A., Meighen E. Differential acylation in vitro with tetradecanoyl coenzyme A and tetradecanoic acid (+ATP) of three polypeptides shown to have induced synthesis in Photobacterium phosphoreum. J Biol Chem. 1984 Feb 10;259(3):1409–1414. [PubMed] [Google Scholar]





