Abstract
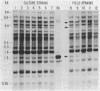
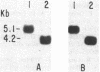
The species of Brucella are very closely related, but Brucella ovis does not express detectable amounts of a protein, designated BCSP31, that is common to the other species. We studied the lack of expression of BCSP31 by Southern analysis. DNAs from the B. ovis culture collection strains and field isolates were probed with a 1.3-kb HindIII fragment encoding BCSP31 of Brucella abortus. The probe hybridized to a 1.6-kb HindIII fragment of all B. ovis strains tested, showing that the gene is present in B. ovis but occurs on a larger restriction fragment. DNA linkage studies and restriction mapping of the cloned polymorphic region of B. ovis showed that the polymorphism was due to a DNA insertion of approximately 0.9 kb at a site downstream of the BCSP31-coding region. When the 1.6-kb polymorphic B. ovis fragment was used to probe a HindIII Southern blot of cellular DNA of strains of B. ovis and of B. abortus, at least 24 fragments of B. ovis and 6 fragments of B. abortus hybridized to the inserted DNA. Specimens of B. ovis collected over a 30-year period on two continents had similar hybridization patterns. The large difference between B. ovis and B. abortus in the number of copies of the repeated DNA is interesting in the context of the closeness of the Brucella species.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allardet-Servent A., Bourg G., Ramuz M., Pages M., Bellis M., Roizes G. DNA polymorphism in strains of the genus Brucella. J Bacteriol. 1988 Oct;170(10):4603–4607. doi: 10.1128/jb.170.10.4603-4607.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B. J., Bills M. M., Egerton J. R., Mattick J. S. Cloning and expression in Escherichia coli of the gene encoding the structural subunit of Bacteroides nodosus fimbriae. J Bacteriol. 1984 Nov;160(2):748–754. doi: 10.1128/jb.160.2.748-754.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUDDLE M. B. Studies on Brucella ovis (n.sp.), a cause of genital disease of sheep in New Zealand and Australia. J Hyg (Lond) 1956 Sep;54(3):351–364. doi: 10.1017/s0022172400044612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey K. M., West D. M. Restriction endonuclease (EcoR1) analysis of Brucella ovis DNA. N Z Vet J. 1987 Oct;35(10):161–162. doi: 10.1080/00480169.1987.35428. [DOI] [PubMed] [Google Scholar]
- Bricker B. J., Tabatabai L. B., Deyoe B. L., Mayfield J. E. Conservation of antigenicity in a 31-kDa Brucella protein. Vet Microbiol. 1988 Dec;18(3-4):313–325. doi: 10.1016/0378-1135(88)90096-x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Mayfield J. E., Bricker B. J., Godfrey H., Crosby R. M., Knight D. J., Halling S. M., Balinsky D., Tabatabai L. B. The cloning, expression, and nucleotide sequence of a gene coding for an immunogenic Brucella abortus protein. Gene. 1988;63(1):1–9. doi: 10.1016/0378-1119(88)90540-9. [DOI] [PubMed] [Google Scholar]
- McGillivery D. J., Webber J. J., Edwards L. D. Restriction endonuclease analysis of Brucella abortus. Res Vet Sci. 1988 Sep;45(2):251–252. [PubMed] [Google Scholar]
- Morris J. A. The use of polyacrylamide gel electrophoresis in taxonomy of Brucella. J Gen Microbiol. 1973 May;76(1):231–237. doi: 10.1099/00221287-76-1-231. [DOI] [PubMed] [Google Scholar]
- Muzny D. M., Ficht T. A., Templeton J. W., Adams L. G. DNA homology of Brucella abortus strains 19 and 2308. Am J Vet Res. 1989 May;50(5):655–661. [PubMed] [Google Scholar]
- Nyman K., Ohtsubo H., Davison D., Ohtsubo E. Distribution of insertion element IS1 in natural isolates of Escherichia coli. Mol Gen Genet. 1983;189(3):516–518. doi: 10.1007/BF00325920. [DOI] [PubMed] [Google Scholar]
- O'Hara M. J., Collins D. M., de Lisle G. W. Restriction endonuclease analysis of Brucella ovis and other Brucella species. Vet Microbiol. 1985 Aug;10(5):425–429. doi: 10.1016/0378-1135(85)90024-0. [DOI] [PubMed] [Google Scholar]
- Ohtsubo H., Nyman K., Doroszkiewicz W., Ohtsubo E. Multiple copies of iso-insertion sequences of IS1 in Shigella dysenteriae chromosome. Nature. 1981 Aug 13;292(5824):640–643. doi: 10.1038/292640a0. [DOI] [PubMed] [Google Scholar]
- Oñate A., Folch H. Comparison of protein components of different species and strains of Brucella. Zentralbl Veterinarmed B. 1989 Jul;36(5):397–400. doi: 10.1111/j.1439-0450.1989.tb00619.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tabatabai L. B., Deyoe B. L., Ritchie A. E. Isolation and characterization of toxic fractions from Brucella abortus. Infect Immun. 1979 Nov;26(2):668–679. doi: 10.1128/iai.26.2.668-679.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verger J. M., Grimont F., Grimont P. A., Grayon M. Taxonomy of the genus Brucella. Ann Inst Pasteur Microbiol. 1987 Mar-Apr;138(2):235–238. doi: 10.1016/0769-2609(87)90199-2. [DOI] [PubMed] [Google Scholar]
- Verstreate D. R., Winter A. J. Comparison of sodium dodecyl sulfate-polyacrylamide gel electrophoresis profiles and antigenic relatedness among outer membrane proteins of 49 Brucella abortus strains. Infect Immun. 1984 Oct;46(1):182–187. doi: 10.1128/iai.46.1.182-187.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. R., Cunningham R. P., Holmes D. S. IST2: an insertion sequence from Thiobacillus ferrooxidans. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7284–7287. doi: 10.1073/pnas.85.19.7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. R., Holmes D. S. Two families of repeated DNA sequences in Thiobacillus ferrooxidans. J Bacteriol. 1987 May;169(5):1861–1870. doi: 10.1128/jb.169.5.1861-1870.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]