Abstract
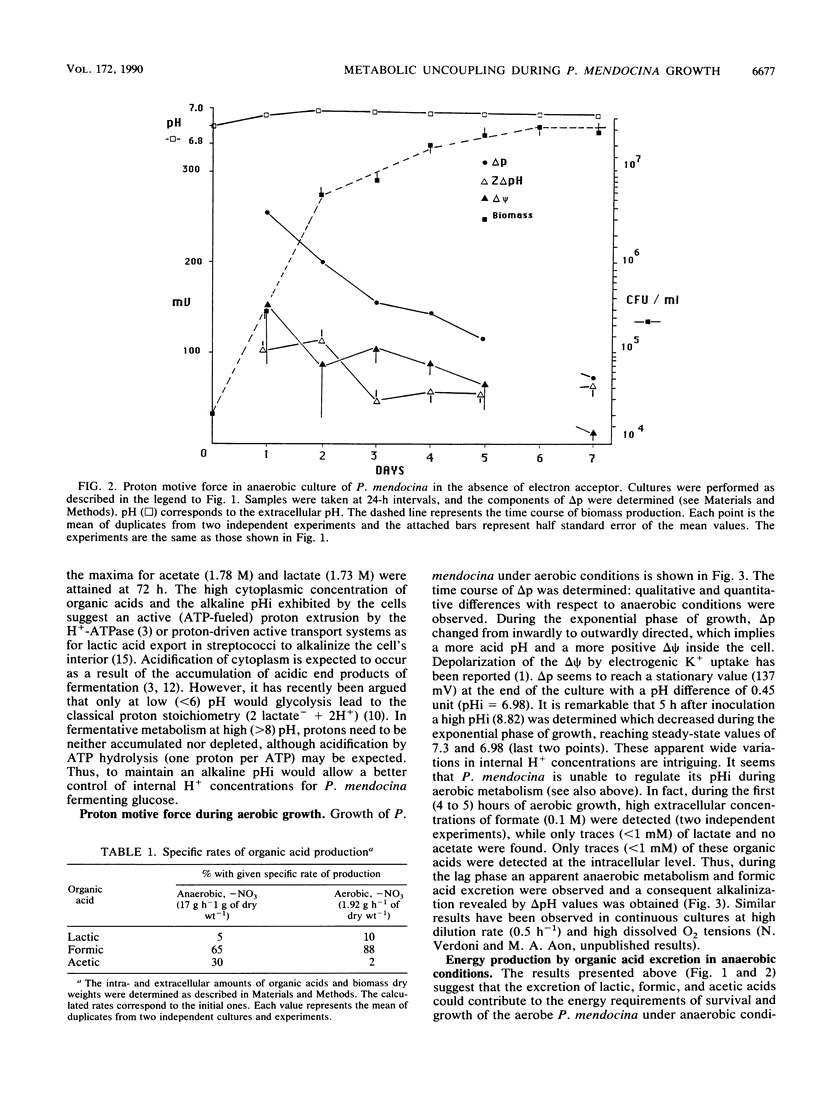
Batch cultures of Pseudomonas mendocina, grown in rich medium with glucose excess, showed metabolic differences dependent upon whether the growth conditions were aerobic or anaerobic, with or without added electron acceptor. Under anaerobic conditions in the absence of nitrate, P. mendocina reached the stationary phase of growth after 2 or 3 days, followed by a stationary phase of 4 to 5 days. Under these conditions, a mixed-type fermentative metabolism (formic, lactic, and acetic acids) appeared. A fivefold-higher specific rate of glucose consumption and eightfold-higher production of organic acids, compared with aerobic cultures, were shown by this microorganism growing anaerobically in the absence of exogenous electron acceptors. The gradients of organic acid produced by P. mendocina under these conditions reached a maximum (lactate, 180 mV; formate, 150 mV; acetate, 215 mV) between days 2 and 3 of culture. The proton motive force (delta p) decreased during growth from -254 to -71 mV. The intracellular pH remained alkaline during the culture, reaching a steady-state value of 7.9. The gradients of organic acids apparently contributed to the generation of a delta p, which, according to the Energy Recycling Model (P. A. M. Michels, J. P. J. Michels, J. Boonstra, and W. N. Konings, FEMS Microbiol. Lett. 5:357-364, 1979), would produce an average energy gain of 1 or 1.5 mol of ATP equivalents per mol of glucose consumed with H+/ATP stoichiometry of 3 or 2, respectively. Low YATP and Yglucose values were observed, suggesting that an uncoupled metabolism exists; i.e., ATP produced by catabolic processes is not directly used for biomass synthesis. This metabolic uncoupling could be induced at least in part by organic acids and the ATP wastage could be induced by a membrane-bound ATPase involved in intracellular pH regulation.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abee T., Hellingwerf K. J., Konings W. N. Effects of potassium ions on proton motive force in Rhodobacter sphaeroides. J Bacteriol. 1988 Dec;170(12):5647–5653. doi: 10.1128/jb.170.12.5647-5653.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baronofsky J. J., Schreurs W. J., Kashket E. R. Uncoupling by Acetic Acid Limits Growth of and Acetogenesis by Clostridium thermoaceticum. Appl Environ Microbiol. 1984 Dec;48(6):1134–1139. doi: 10.1128/aem.48.6.1134-1139.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth I. R. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985 Dec;49(4):359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. A. Metabolism of methylglyoxal in microorganisms. Annu Rev Microbiol. 1984;38:49–68. doi: 10.1146/annurev.mi.38.100184.000405. [DOI] [PubMed] [Google Scholar]
- Hochachka P. W. Defense strategies against hypoxia and hypothermia. Science. 1986 Jan 17;231(4735):234–241. doi: 10.1126/science.2417316. [DOI] [PubMed] [Google Scholar]
- Hochachka P. W., Mommsen T. P. Protons and anaerobiosis. Science. 1983 Mar 25;219(4591):1391–1397. doi: 10.1126/science.6298937. [DOI] [PubMed] [Google Scholar]
- Hueting S., Tempest D. W. Influence of acetate on the growth of Candida utilis in continuous culture. Arch Microbiol. 1977 Oct 24;115(1):73–78. doi: 10.1007/BF00427848. [DOI] [PubMed] [Google Scholar]
- Lessie T. G., Phibbs P. V., Jr Alternative pathways of carbohydrate utilization in pseudomonads. Annu Rev Microbiol. 1984;38:359–388. doi: 10.1146/annurev.mi.38.100184.002043. [DOI] [PubMed] [Google Scholar]
- Nelson N., Taiz L. The evolution of H+-ATPases. Trends Biochem Sci. 1989 Mar;14(3):113–116. doi: 10.1016/0968-0004(89)90134-5. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Schuldiner S. pH homeostasis in bacteria. Biochim Biophys Acta. 1981 Dec;650(2-3):151–166. doi: 10.1016/0304-4157(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H., Bettenhaussen C. W. A continuous culture study of an ATPase-negative mutant of Escherichia coli. Arch Microbiol. 1977 Jun 20;113(3):185–189. doi: 10.1007/BF00492023. [DOI] [PubMed] [Google Scholar]
- Tempest D. W., Neijssel O. M. The status of YATP and maintenance energy as biologically interpretable phenomena. Annu Rev Microbiol. 1984;38:459–486. doi: 10.1146/annurev.mi.38.100184.002331. [DOI] [PubMed] [Google Scholar]
- Wenner C. E. Pasteur and Crabtree effects--assay in cells. Methods Enzymol. 1979;55:289–297. doi: 10.1016/0076-6879(79)55033-2. [DOI] [PubMed] [Google Scholar]
- den Hollander J. A., Ugurbil K., Brown T. R., Shulman R. G. Phosphorus-31 nuclear magnetic resonance studies of the effect of oxygen upon glycolysis in yeast. Biochemistry. 1981 Sep 29;20(20):5871–5880. doi: 10.1021/bi00523a034. [DOI] [PubMed] [Google Scholar]
- ten Brink B., Konings W. N. Electrochemical proton gradient and lactate concentration gradient in Streptococcus cremoris cells grown in batch culture. J Bacteriol. 1982 Nov;152(2):682–686. doi: 10.1128/jb.152.2.682-686.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


