Abstract
In addition to its role as an inhibitory neurotransmitter, γ-aminobutyric acid (GABA) is presumed to be involved in the development and plasticity of the nervous system. GABA is synthesized by glutamic acid decarboxylase (GAD), but the respective roles of its two isoforms (GAD65 and 67) have not been determined. The selective elimination of each GAD isoform by gene targeting is expected to clarify these issues. Recently we have produced GAD65 −/− mice and demonstrated that lack of GAD65 does not change brain GABA contents or animal behavior, except for a slight increase in susceptibility to seizures. Here we report the production of GAD67 −/− mice. These mice were born at the expected frequency but died of severe cleft palate during the first morning after birth. GAD activities and GABA contents were reduced to 20% and 7%, respectively, in the cerebral cortex of the newborn GAD67 −/− mice. Their brain, however, did not show any discernible defects. Previous pharmacological and genetic investigations have suggested the involvement of GABA in palate formation, but this is the first demonstration of a role for GAD67-derived GABA in the development of nonneural tissue.
γ-Aminobutyric acid (GABA) is the principal inhibitory neurotransmitter (1, 2) that is synthesized from glutamic acid by glutamic acid decarboxylase (GAD) in GABA-utilizing (GABAergic) neurons (3, 4). Recent investigations have revealed that GAD and GABA are also transiently expressed in non-GABAergic cells of the embryonic and adult nervous system (5–7), suggesting their involvement in development and plasticity (8–10). GAD exists as two isoforms with molecular masses of 65 and 67 kDa (GAD65 and GAD67, respectively), which are encoded by independent genes (4). Several different properties of these isoforms (3, 4, 11) have suggested that they have distinct roles in neural functions. The use of gene targeting for the selective elimination of each GAD isoform is expected to settle these issues. The recent production of GAD65 −/− mice (12, 13) yielded the unexpected finding that lack of GAD65 does not change brain GABA contents or animal behavior, except for a slight increase in susceptibility to seizures. Here we report the production of GAD67 −/− mice. These mice have cleft palate, resulting in neonatal death, and a marked reduction of GABA in the absence of discernible structural defects in the brain.
MATERIALS AND METHODS
Construction of the Targeting Vector and Production of Mutant Mice.
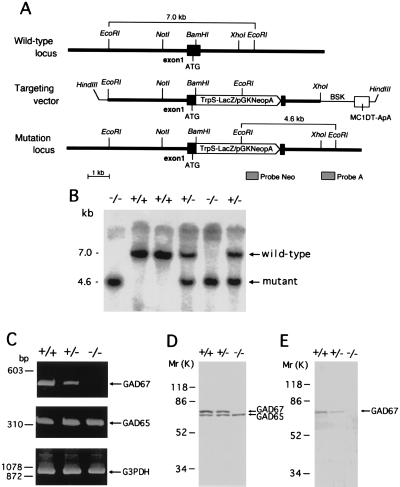
GAD67 gene fragments were cloned from a TT2 cell genomic library (14) using mouse GAD67 cDNA (15) as the probe. A 7.0-kb EcoRI-EcoRI fragment containing exon 1 was subcloned into pBS (Stratagene). A TrpS-LacZ/pGKNeo cassette (16) was inserted into a unique BamHI site in exon 1. Diphtheria toxin-A gene (pMC1DT-ApA) was included in the targeting vector to allow selection against random integration (12). The targeting vector (Fig. 1A) was linearized with HindIII and electroporated into TT2 embryonic stem (ES) cells (16). DNA isolated from G418 (GIBCO/BRL)-resistant clones was digested with EcoRI and screened by Southern blot analysis using the external probe A. Southern blot analysis was also carried out using a Neo probe to ensure the selection of ES clones containing only one copy of the construct. Homologous recombination was detected in 4 of 96 clones analyzed. These ES cells were injected into eight-cell embryos from ICR mice. The mutant mice were obtained by mating chimeric mice with C57BL/6 mice, as described previously (12, 16). The day when the vaginal plug appeared in the dam was designated as embryonic day 0.5 (E0.5), and the morning following birth was designated as postnatal day 0.5 (P0.5).
Figure 1.
Targeted disruption of the mouse GAD67 gene. (A) Schematic representation of mouse GAD67 genomic DNA, the targeting vector, and the disrupted gene. A TrpSLacZ/pGKNeo cassette was inserted into the BamHI site of exon 1 located downstream from the translation initiation codon ATG. The location of probes A and Neo that were used for Southern blot analysis are indicated. (B) Southern blot analysis of tail DNA isolated from P0.5 mouse littermates following digestion with EcoRI and probing with the external probe A. (C) RT-PCR analysis of GAD67 (495 bp) and GAD65 transcripts (355 bp) from P0.5 mouse cerebral cortex. RT-PCR of G3PDH mRNA was used to assess the quality of the mRNA. (D and E) Western blot analysis of P0.5 mouse cerebral cortex using anti-GAD65/67 (D) and anti-GAD67 (E) antibodies.
Reverse Transcriptase–PCR (RT-PCR) Analysis.
Total RNA was prepared from the cerebral cortex of P0.5 mice using TRIzol Reagent (GIBCO/BRL). RNA was reverse transcribed and PCR amplified by using GAD67 5′ and 3′ primers [corresponding to nucleotides 282–303 and 759–777 of GAD67 cDNA (15), respectively] or GAD65 5′ and 3′ primers [corresponding to nucleotides 921–940 and 1257–1276 of the GAD65 cDNA (12), respectively]. Glyceraldehyde-3-phosphate dehydrogenase (G3PDH) primers were used to assess the quality of the RNA (17). The 495-, 355- and 983-bp fragments are derived from GAD67, GAD65, and G3PDH mRNA, respectively.
Western Blot Analysis.
The analysis was performed as previously described (12). Anti-GAD65/67 antiserum (12) recognized both GAD65 and 67, and anti-GAD67 antiserum (K2, Chemicon) selectively detected GAD67.
Measurement of GAD Activity and GABA Content.
The enzymatic activity of GAD in the tissue homogenates was assayed by conversion of 14C-labeled glutamic acid to 14CO2 in the presence or absence of 200 μM pyridoxal phosphate (PLP) as previously described with slight modifications (12, 18). GABA levels in the tissue homogenates were measured using high-performance liquid chromatography and fluorescence detection of o-phtalaldehyde-derivatized adducts (BAS, Japan).
In Situ Hybridization.
Tissues were quickly frozen in powdered dry ice, and frozen sections of 10–12 μm thickness were prepared. In situ hybridization was performed using digoxigenin-labeled GAD67 or 65 cRNA probe as previously described (19). Digoxigenin was detected with anti-digoxigenin antibody conjugated with alkaline phosphatase. Counterstaining was performed with 0.2% methyl green.
Immunohistochemistry.
Brains of P0.5 mice or whole embryos (E14.5 and E17.5) were fixed by immersion in 4% paraformaldehyde and 0.25% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4). After equilibration with 30% sucrose solution, 10 μm frozen sections were prepared. Immunohistochemistry with anti-GABA antiserum (A-2052, Sigma) was performed using Vectastain ABC kit (Vector Laboratories). Staining with hematoxylin-eosin or 0.1% cresyl violet was also performed on the frozen sections.
RESULTS AND DISCUSSION
Production of Mutant Mice.
We generated GAD67 mutant mice from TT2 ES cells in which the first exon of GAD67 gene had been disrupted by insertion of a LacZ-Neo cassette (Fig. 1A). Deletion of the GAD67 gene in mice derived from these cells was confirmed by Southern blot analysis of tail-tissue DNA (Fig. 1B). RT-PCR analysis revealed that GAD67 mRNA was not expressed in the brains of GAD67 −/− mice and was reduced to about one-half normal levels in GAD67 +/− brain (Fig. 1C). There were no differences in GAD65 mRNA among GAD67 +/+, +/−, and −/− mice (Fig. 1C). Corresponding changes were observed in GAD67 protein by Western blot analysis (Fig. 1 D and E).
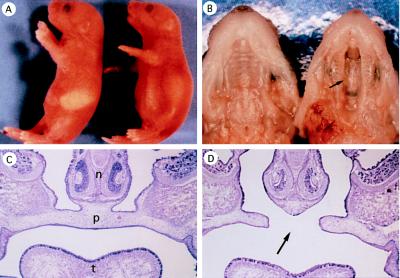
Mice were produced from heterozygous parents at the expected Mendelian frequency (24% wild type, 51% heterozygous, and 25% homozygous; n = 160). At birth, the littermates were indistinguishable from each other in appearance. Afterward, however, homozygous mutant mice displayed gradual swelling of the bowels and their stomachs remained unfilled with milk (Fig. 2A). They became cyanotic and exhibited gasping respirations. All GAD67 −/− mice died during the first morning after birth (day P0.5). Severe cleft palate was found in all homozygous mutants (Figs. 2 B and D and 3B). Formation of the second palate appeared to be completely obstructed. The stomach, duodenum, and jejunum of the null mutants were filled with swallowed air. The lung was partially airated, but was mostly atelectatic (not shown). When milk was introduced to the mouth with a cannula, the newborn mice with cleft palate showed the similar suckling response to that of wild mice. Therefore, it was tentatively concluded that the neonatal death was due to respiratory failure rather than to the impairment of suckling.
Figure 2.
GAD67 knockout mice and cleft palate. (A) Lateral view of wild-type (Left) and GAD67 −/− mutant (Right) newborn mice (P0.5). In homozygous mutants the abdomen is distended but milk is not observed in the stomach. (B) Ventral view of the upper jaw of wild-type (Left) and GAD67 −/− mutant (Right) newborns. Cleft palate (arrows in B and D) was observed in all homozygous mutants. (C and D) Coronal sections of E17.5 facial region of wild-type (C) and GAD67 −/− (D) mice. n, Nasal septa; p, palate; t, tongue.
GAD Activity and GABA Content in the Cerebral Cortex.
The results on the P0.5 and adult mice are summarized in Table 1. Because only data for adult GAD65 mutant mice were reported in our previous paper (12), the values for GAD65 −/− mice at P0.5 have been included in the table. In the cerebral cortex of P0.5 GAD67 −/− mice, GAD activity was less than 20% and GABA content was 7% of the respective values for wild-type littermates (Table 1). GAD67 +/− cerebral cortex contained 62% PLP-independent GAD activity and 65% GABA content of wild-type cortex, but the reduction was still highly significant. Comparable reductions were also observed in E14.5 GAD67 homozygous and heterozygous mutants (data not shown) and in GAD67 +/− adults. The marked reductions of GABA in GAD67 mutants are in sharp contrast to GAD65 knockout mice, which maintain normal levels of GABA (Table 1 and ref. 12). These results indicate that the major component of GAD in developing animals is GAD67. Selective reductions of PLP-dependent GAD activity in GAD65 −/− mice are also shown by the decrease in the ratio of PLP-dependent to PLP-independent activity (Table 1 and ref. 12). These findings are consistent with the view that dependency of GAD activity on exogenous PLP is higher for GAD65 than for GAD67 (3, 4). The significant reductions of not only PLP-independent but also dependent activities in P0.5 GAD67 mutants, however, indicate considerable PLP dependency for GAD67.
Table 1.
GAD activity and GABA content in the cerebral cortex of newborn (P0.5) and adult mice
| Genotype | GAD activity, nmol 14CO2 per 2 h/mg protein
|
Ratio
|
GABA content, nmol/mg protein | |
|---|---|---|---|---|
| −PLP* | +PLP* | +PLP/−PLP | ||
| P0.5 | ||||
| GAD65+/+ 67−/− | 1.39 ± 0.08‡ | 4.00 ± 0.73‡ | 2.88 (6) | 0.32 ± 0.05 ‡ (6) |
| GAD65+/+ 67+/− | 5.64 ± 0.41‡ | 16.99 ± 1.06‡ | 3.01 (8) | 2.95 ± 0.17 ‡ (8) |
| GAD65−/− 67+/+ | 8.82 ± 0.73 | 12.43 ± 1.22‡ | 1.40† (4) | 3.97 ± 0.16 (4) |
| GAD65+/+ 67+/+ | 9.15 ± 0.41 | 20.58 ± 1.39 | 2.25 (6) | 4.54 ± 0.10 (6) |
| Adult | ||||
| GAD65+/+ 67+/− | 44.36 ± 2.04† | 129.17 ± 5.64 | 2.91 (6) | 12.51 ± 0.79† (6) |
| GAD65+/+ 67+/+ | 58.91 ± 7.19 | 146.00 ± 8.17 | 2.47 (4) | 15.90 ± 0.60 (4) |
Values are expressed as the mean ± SEM. Numbers of mice (from several littermates) are indicated in parentheses.
Absence or presence of 200 μM pyridoxal 5′-phosphate in the incubation medium, respectively.
P < 0.05 and
P < 0.01, compared with GAD65+/+GAD67+/+ (Student’s t test).
Histology and Behavior.
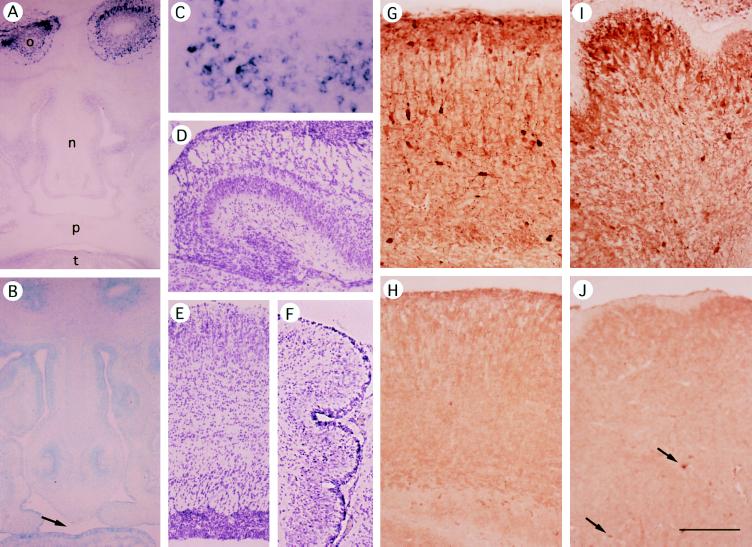
In spite of the proposed neurogenetic role of GABA (6, 8–10), the gross structure of the brain appeared normal in the GAD67 −/− newborn (Fig. 3 D–F). The cerebral cortex had the usual pattern of cell distribution in each layer (Fig. 3E), and the hippocampus and cerebellum showed characteristic histologies (Fig. 3 D and F). As expected, in situ hybridization of GAD67 −/− brain demonstrated the absence of GAD67 mRNA signals, without significant changes in GAD65 signals (not shown). Immunohistochemistry with anti-GABA antibody disclosed that GABA staining was greatly reduced in the cerebral cortex, cerebellum, and most other regions (Fig. 3 G–J) but the reduction was not so pronounced in other regions such as the superior colliculus and brainstem. In the GAD67 −/− cerebellum (Fig. 3J), most cortical GABAergic neurons did not show GABA immunoreactivities, but a few cells (arrows) and their processes in the deep part were highly positive. If GABA is required during the early stage of neurogenesis (6, 8, 9), the small amount of GABA synthesized by GAD65 may be sufficient to allow normal or nearly normal development of the GAD67 −/− brain. This hypothesis will be explored using GAD65 and GAD67 double-knockout mice that completely lack GABA.
Figure 3.
Expression of GAD67 mRNA and GABA in GAD67 −/− and +/+ brain and head region. (A–C) In situ hybridization of frontal sections of E14.5 mouse heads using a GAD67 probe. (A and C) GAD67 +/+. o, olfactory bulb; n, nasal septa; p, palate; t, tongue. C shows the olfactory bulb at higher magnification. (B) GAD67 −/−; counterstained with methyl green. GAD67 signals are found only in neural tissue (olfactory bulb) of GAD67+/+ or +/− mice. (D–F) Hematoxylin-eosin staining of P0.5 GAD67 −/− brain. (D) Hippocampus. (E) Cerebral cortex. (F) Cerebellum. (G–J) Immunohistochemistry with anti-GABA antibody (Sigma). (G and H) Cerebral cortex. (I and J) Cerebellum. (G and I) P0.5 wild-type mice. (H and J) P0.5 null mutant mice. Arrows in J indicate GABA-positive neurons in the deep part of GAD67 −/− cerebellum. (Bar in J = 500 μm for A, B, and D–F; 200 μm for G–J; and 100 μm for C.)
Movement of the limbs and trunk in newborn GAD67 null mutants was active and coordinated without apparent neurological disorders. After delivery by cesarean section at E17.5, fetuses of all genotypes commenced body movements similarly. GABAergic synapses are already formed in newborn animals, but may not exert inhibitory functions because of high intracellular concentrations of chloride (20, 21). For these reasons, GABA deficiency may not result in behavioral abnormalities in newborn mice. Decreases in GAD activity and GABA content in the brain were also detected in adult GAD67 +/− mutants (Table 1). In these animals, however, neurological disorders were not observed, and learning in a Morris water maze and susceptibility to picrotoxin-induced seizures did not differ from those of wild-type mice (data not shown).
Cleft Palate and Analysis on Palatal Tissue.
All of GAD67 −/− mice died of cleft palate during the first morning after birth. Because palate development in mice takes place during day E13.5 to E15.5 (22), we examined expression of GAD and GABA in the maxillary regions, including the palate, in E14.5 mice. Wee et al. (23) have shown that the GABA content of the palate is about half of that in the body excluding the brain, and is lower on day E14 than on E13 and E15. GAD activity and GABA were almost undetectable biochemically, but Western blot analysis showed a faint but clear band of GAD67 protein in the fetal maxillary tissue in GAD67 +/+ and +/− mice (data not shown). This band was absent in −/− mice. Neither in situ hybridization (Fig. 3B) nor immunohistochemistry demonstrated GAD- or GABA-positive cells in E14.5 palates.
The occurrence of cleft palate in the absence of apparent defects in brain morphogenesis in GAD67 knockout mice was unexpected. Previous pharmacological and genetic investigations, however, have indicated the involvement of GABA in palate. Administration of the GABA potentiator diazepam to pregnant mice induces cleft palate in their offspring (24). GABA application to E14.5 mouse embryos in short-term culture also inhibits palate development (25). Furthermore, cleft palate and neurological disorders are observed in pink-eyed cleft palate (p) mutant mice, which are thought to be defective in the β3 subunit of GABAA receptor (26, 27). As indicated above, the GABAA receptor β3 subunit is required for palate formation, but the expression of this receptor in the palate has not yet been described. At present, it is not known whether GABA functions directly in palate formation or indirectly by affecting another part of the central nervous system. It is also not known whether GAD67 itself plays a specific role in the development of the palate. In vitro experiments on isolated cleft tissue (28) may provide clues to answer these questions.
Growth factors such as transforming growth factor β and epidermal growth factor, intracellular signal transduction pathways involving protein kinases, and interactions between epithelial and mesenchymal cells have been investigated in connection with palate and craniofacial development (22, 29). Cleft palate has also been shown to be induced by a variety of teratogens, including halogenated aromatic hydrocarbons (29). How GABA is involved in the signaling cascades that function during palatal formation remains to be investigated.
Although GABA is generally regarded as the neurotransmitter or regulator of cell function, it can also be the substrate to be oxidized in ATP-generating tricarboxylic acid cycle in mitochondria (30). The GAD-deficient mice should be useful for the study of this role of GABA.
It has been proposed that GAD65 participates more actively in nervous system function than GAD67, because GAD65 appears to be concentrated in the nerve terminals while GAD67 is not (3, 4, 11). The results of our current and previous investigations (12) indicate, however, that GAD67, but not GAD65, substantially controls GABA levels and that GABA synthesized by GAD67 is involved in the development of the palate.
Acknowledgments
We thank S. Furuya for histological studies and D. Saffen for critical reading of the manuscript. This work was supported by Grants-in-Aid from the Ministry of Education, Science, Sports, and Culture (Japan).
ABBREVIATIONS
- GABA
γ-aminobutyric acid
- GAD
glutamic acid decarboxylase
- PLP
pyridoxal phosphate
- ES cell
embryonic stem cell
- RT-PCR
reverse transcriptase–PCR
References
- 1.Obata K. Int Rev Neurobiol. 1972;15:167–187. doi: 10.1016/s0074-7742(08)60330-x. [DOI] [PubMed] [Google Scholar]
- 2.Roberts E, Chase T N, Tower D B, editors. GABA in Nervous System Function. New York: Raven; 1976. [Google Scholar]
- 3.Erlander M G, Tobin A J. Neurochem Res. 1991;16:215–226. doi: 10.1007/BF00966084. [DOI] [PubMed] [Google Scholar]
- 4.Erlander M G, Tillakaratne N J K, Feldblum S, Patel N, Tobin A J. Neuron. 1991;7:91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- 5.Ma W, Chang L, Zhang L, Barker J L. J Neurosci. 1995;15:2547–2560. doi: 10.1523/JNEUROSCI.15-03-02547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LoTurco J J, Owens D F, Heath M J S, Davis M B E, Kriegstein A R. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 7.Sloviter R S, Dichter M A, Rachinsky T L, Dean E, Goodman J H, Sollas A L, Martin D L. J Comp Neurol. 1996;373:593–618. doi: 10.1002/(SICI)1096-9861(19960930)373:4<593::AID-CNE8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 8.Behar T N, Schaffner A E, Colton C A, Somogyi R, Olah Z, Lehel C, Barker J L. J Neurosci. 1994;14:29–38. doi: 10.1523/JNEUROSCI.14-01-00029.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbin G, Pollard H, Gaiarsa J L, Ben-Ari Y Y. Neurosci Lett. 1993;152:150–154. doi: 10.1016/0304-3940(93)90505-f. [DOI] [PubMed] [Google Scholar]
- 10.Redburn D A, Schousboe A, editors. Neurotrophic Activity of GABA During Development. New York: Liss; 1987. [Google Scholar]
- 11.Dirkx R, Jr, Thomas A, Li L, Lernmark A, Sherwin R S, De Camili P, Solimena M. J Biol Chem. 1995;270:2241–2246. doi: 10.1074/jbc.270.5.2241. [DOI] [PubMed] [Google Scholar]
- 12.Asada H, Kawamura Y, Maruyama K, Kume K, Ding R-G, Ji F Y, Kanbara N, Kuzume H, Sanbo M, Yagi T, Obata K. Biochem Biophys Res Commun. 1996;229:891–895. doi: 10.1006/bbrc.1996.1898. [DOI] [PubMed] [Google Scholar]
- 13.Kash S, Johnson R, Tecott L, Lowenstein D, Hanahan D, Baekkeskov S. Soc Neurosci Abstr. 1996;22:1295. [Google Scholar]
- 14.Yagi T, Nada S, Watanabe N, Tamemoto H, Kohmura N, Ikawa Y, Aizawa S. Anal Biochem. 1993;214:77–86. doi: 10.1006/abio.1993.1459. [DOI] [PubMed] [Google Scholar]
- 15.Katarova Z, Szabo G, Mugnaini E, Greenspan R J. Eur J Neurosci. 1990;2:190–202. doi: 10.1111/j.1460-9568.1990.tb00412.x. [DOI] [PubMed] [Google Scholar]
- 16.Yagi T, Aizawa S, Tokunaga T, Shigetani Y, Takeda N, Ikawa Y. Nature (London) 1993;366:742–745. doi: 10.1038/366742a0. [DOI] [PubMed] [Google Scholar]
- 17.Tso J Y, Sun X H, Kao T H, Reece K S, Wu R. Nucleic Acids Res. 1985;13:2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baekkeskov S. Nature (London) 1990;347:151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto T, Obata K. Neurosci Res. 1991;12:514–527. doi: 10.1016/s0168-0102(09)80004-7. [DOI] [PubMed] [Google Scholar]
- 20.Luhmann H J, Prince D A. J Neurophysiol. 1991;65:247–263. doi: 10.1152/jn.1991.65.2.247. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Spiegelman I, Carlen P L. J Physiol (London) 1991;444:25–49. doi: 10.1113/jphysiol.1991.sp018864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferguson, M. W. J. (1988) Development (Cambridge, U.K.) 103, Suppl., 41–60. [DOI] [PubMed]
- 23.Wee E L, Norman E J, Zimmerman E F. J Craniofac Genet Dev Biol. 1986;6:53–61. [PubMed] [Google Scholar]
- 24.Miller R P, Becker B A. Toxicol Appl Pharmacol. 1975;32:53–61. doi: 10.1016/0041-008x(75)90194-5. [DOI] [PubMed] [Google Scholar]
- 25.Wee E L, Zimmerman E F. Teratology. 1983;28:15–22. doi: 10.1002/tera.1420280104. [DOI] [PubMed] [Google Scholar]
- 26.Culiat C T, Stubbs L, Nicholls R D, Montgomery C S, Russell L B, Johnson D K, Rinchik E M. Proc Natl Acad Sci USA. 1993;90:5105–5109. doi: 10.1073/pnas.90.11.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Culiat C T, Stubbs L J, Woychik R P, Russell L B, Johnson D K, Rinchik E M. Nat Genet. 1995;11:344–346. doi: 10.1038/ng1195-344. [DOI] [PubMed] [Google Scholar]
- 28.Brunet C L, Sharpe P M, Ferguson M W J. Int J Dev Biol. 1995;39:345–355. [PubMed] [Google Scholar]
- 29.Greene R M. Crit Rev Toxicol. 1989;20:137–152. doi: 10.3109/10408448909017907. [DOI] [PubMed] [Google Scholar]
- 30.Baxter C F. In: GABA in Nervous System Function. Roberts E, Clause T N, Tower D B, editors. New York: Raven; 1976. pp. 61–87. [Google Scholar]