Abstract
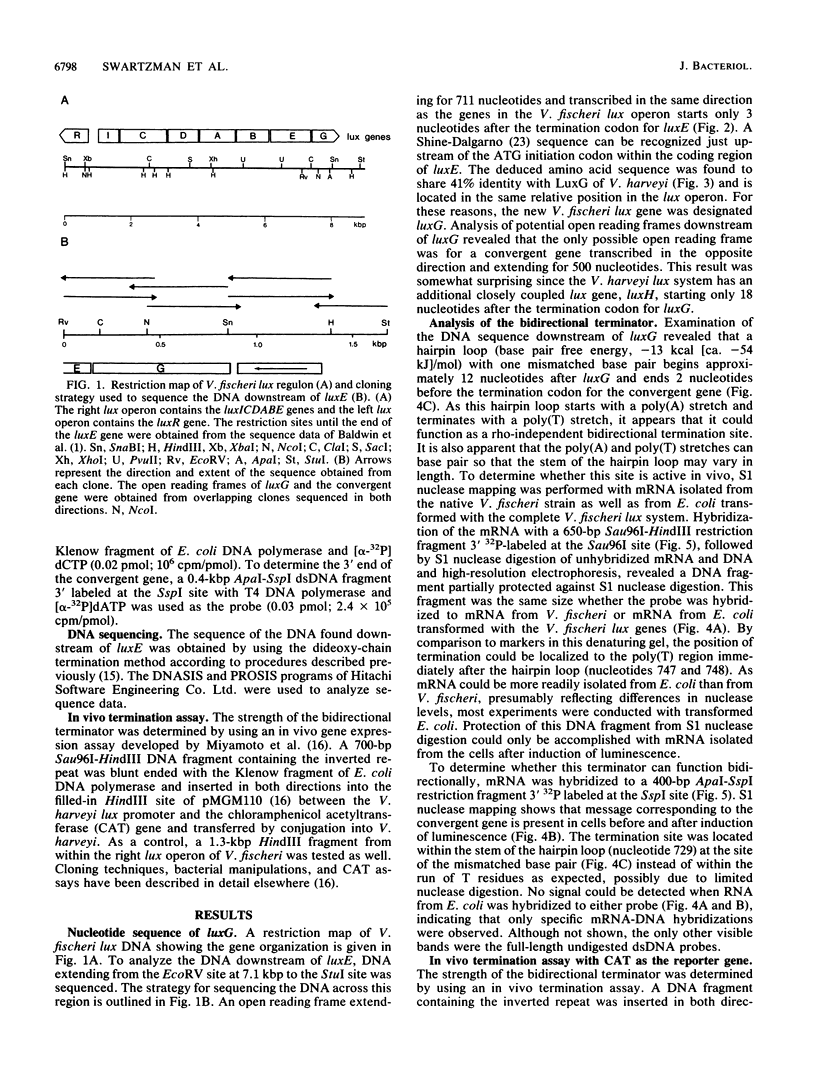
The DNA downstream of the lux structural genes in the Vibrio fischeri lux operon has been sequenced and a new lux gene (luxG) has been identified. A hairpin loop that begins with a poly(A) region and ends with a poly(T) region and thus can function as a bidirectional termination site for luxG and a convergent gene is located immediately downstream of luxG. 3' S1 nuclease mapping has demonstrated that the luxG mRNA was induced in a cell-density-dependent fashion consistent with it being part of the lux system and that the lux mRNA terminated immediately after the hairpin loop. The mRNA coded by an open reading frame convergent to luxG on the complementary strand was also shown by S1 nuclease mapping to overlap the lux mRNA for at least 20 nucleotides before termination. Expression of DNA containing the hairpin loop, placed between a strong promoter and a reporter gene and transferred by conjugation into luminescent bacteria, demonstrated the very high efficiency of termination by this hairpin loop oriented in either direction. These results also demonstrate that the organization of the genes at the 3' ends of the lux operons of V. fischeri and V. harveyi has clearly diverged.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boylan M., Graham A. F., Meighen E. A. Functional identification of the fatty acid reductase components encoded in the luminescence operon of Vibrio fischeri. J Bacteriol. 1985 Sep;163(3):1186–1190. doi: 10.1128/jb.163.3.1186-1190.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlomagno M. S., Riccio A., Bruni C. B. Convergently functional, Rho-independent terminator in Salmonella typhimurium. J Bacteriol. 1985 Jul;163(1):362–368. doi: 10.1128/jb.163.1.362-368.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N. Y., Hu F. M., Paulus H. Nucleotide sequence of the overlapping genes for the subunits of Bacillus subtilis aspartokinase II and their control regions. J Biol Chem. 1987 Jun 25;262(18):8787–8798. [PubMed] [Google Scholar]
- Christianson T. W., Clayton D. A. In vitro transcription of human mitochondrial DNA: accurate termination requires a region of DNA sequence that can function bidirectionally. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6277–6281. doi: 10.1073/pnas.83.17.6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap P. V., Greenberg E. P. Control of Vibrio fischeri lux gene transcription by a cyclic AMP receptor protein-luxR protein regulatory circuit. J Bacteriol. 1988 Sep;170(9):4040–4046. doi: 10.1128/jb.170.9.4040-4046.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engebrecht J., Nealson K., Silverman M. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983 Mar;32(3):773–781. doi: 10.1016/0092-8674(83)90063-6. [DOI] [PubMed] [Google Scholar]
- Engebrecht J., Silverman M. Identification of genes and gene products necessary for bacterial bioluminescence. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4154–4158. doi: 10.1073/pnas.81.13.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson G. S., Davidson B. E. Nucleotide sequence and transcription of the phenylalanine and tyrosine operons of Escherichia coli K12. J Mol Biol. 1984 Dec 25;180(4):1023–1051. doi: 10.1016/0022-2836(84)90269-9. [DOI] [PubMed] [Google Scholar]
- Jerlström P. G., Bezjak D. A., Jennings M. P., Beacham I. R. Structure and expression in Escherichia coli K-12 of the L-asparaginase I-encoding ansA gene and its flanking regions. Gene. 1989 May 15;78(1):37–46. doi: 10.1016/0378-1119(89)90312-0. [DOI] [PubMed] [Google Scholar]
- Lloubès R., Baty D., Lazdunski C. Transcriptional terminators in the caa-cal operon and cai gene. Nucleic Acids Res. 1988 May 11;16(9):3739–3749. doi: 10.1093/nar/16.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini J. A., Boylan M., Soly R. R., Graham A. F., Meighen E. A. Cloning and expression of the Photobacterium phosphoreum luminescence system demonstrates a unique lux gene organization. J Biol Chem. 1988 Oct 5;263(28):14308–14314. [PubMed] [Google Scholar]
- Maniatis T., Efstratiadis A. Fractionation of low molecular weight DNA or RNA in polyacrylamide gels containing 98% formamide or 7 M urea. Methods Enzymol. 1980;65(1):299–305. doi: 10.1016/s0076-6879(80)65040-x. [DOI] [PubMed] [Google Scholar]
- Miyamoto C. M., Boylan M., Graham A. F., Meighen E. A. Organization of the lux structural genes of Vibrio harveyi. Expression under the T7 bacteriophage promoter, mRNA analysis, and nucleotide sequence of the luxD gene. J Biol Chem. 1988 Sep 15;263(26):13393–13399. [PubMed] [Google Scholar]
- Miyamoto C. M., Graham A. F., Meighen E. A. Nucleotide sequence of the LuxC gene and the upstream DNA from the bioluminescent system of Vibrio harveyi. Nucleic Acids Res. 1988 Feb 25;16(4):1551–1562. doi: 10.1093/nar/16.4.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto C. M., Meighen E. A., Graham A. F. Transcriptional regulation of lux genes transferred into Vibrio harveyi. J Bacteriol. 1990 Apr;172(4):2046–2054. doi: 10.1128/jb.172.4.2046-2054.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA). Proc Natl Acad Sci U S A. 1984 Apr;81(7):1966–1970. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal R. J., Chater K. F. Nucleotide sequence analysis reveals similarities between proteins determining methylenomycin A resistance in Streptomyces and tetracycline resistance in eubacteria. Gene. 1987;58(2-3):229–241. doi: 10.1016/0378-1119(87)90378-7. [DOI] [PubMed] [Google Scholar]
- Paces V., Vlcek C., Urbánek P. Nucleotide sequence of the late region of Bacillus subtilis phage PZA, a close relative of phi 29. Gene. 1986;44(1):107–114. doi: 10.1016/0378-1119(86)90048-x. [DOI] [PubMed] [Google Scholar]
- Platt T. Transcription termination and the regulation of gene expression. Annu Rev Biochem. 1986;55:339–372. doi: 10.1146/annurev.bi.55.070186.002011. [DOI] [PubMed] [Google Scholar]
- Postle K., Good R. F. A bidirectional rho-independent transcription terminator between the E. coli tonB gene and an opposing gene. Cell. 1985 Jun;41(2):577–585. doi: 10.1016/s0092-8674(85)80030-1. [DOI] [PubMed] [Google Scholar]
- Schollmeier K., Gärtner D., Hillen W. A bidirectionally active signal for termination of transcription is located between tetA and orfL on transposon Tn10. Nucleic Acids Res. 1985 Jun 25;13(12):4227–4237. doi: 10.1093/nar/13.12.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soly R. R., Mancini J. A., Ferri S. R., Boylan M., Meighen E. A. A new lux gene in bioluminescent bacteria codes for a protein homologous to the bacterial luciferase subunits. Biochem Biophys Res Commun. 1988 Aug 30;155(1):351–358. doi: 10.1016/s0006-291x(88)81092-1. [DOI] [PubMed] [Google Scholar]
- Swartzman E., Miyamoto C., Graham A., Meighen E. Delineation of the transcriptional boundaries of the lux operon of Vibrio harveyi demonstrates the presence of two new lux genes. J Biol Chem. 1990 Feb 25;265(6):3513–3517. [PubMed] [Google Scholar]
- Westhoff P., Herrmann R. G. Complex RNA maturation in chloroplasts. The psbB operon from spinach. Eur J Biochem. 1988 Feb 1;171(3):551–564. doi: 10.1111/j.1432-1033.1988.tb13824.x. [DOI] [PubMed] [Google Scholar]