Abstract
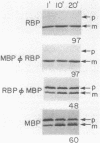
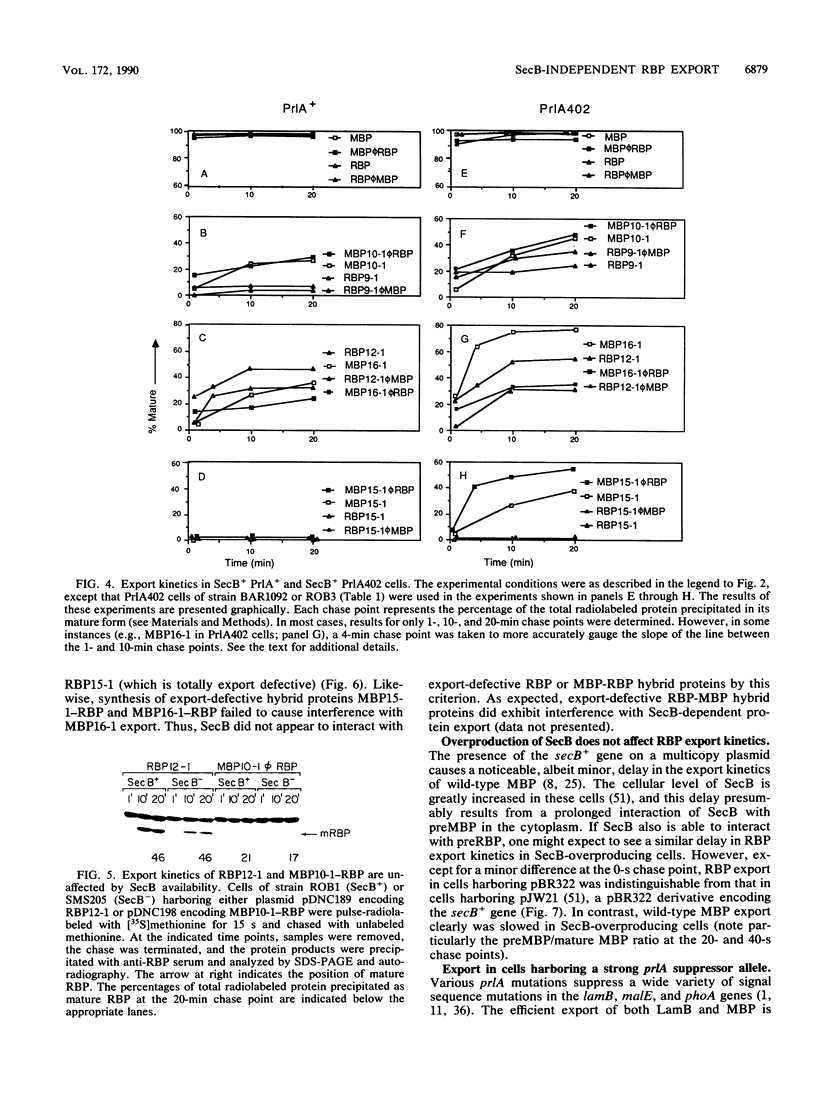
The efficient export of the Escherichia coli maltose-binding protein (MBP) is known to be SecB dependent, whereas ribose-binding protein (RBP) export is SecB independent. When the MBP and RBP signal peptides were exchanged precisely at the signal peptidase processing sites, the resultant RBP-MBP and MBP-RBP hybrid proteins both were efficiently exported in SecB+ cells. However, only MBP-RBP was efficiently exported in SecB- cells; RBP-MBP exhibited a significant export defect, a finding that was consistent with previous proposals that SecB specifically interacts with the mature moiety of precursor MBP to promote export. The relatively slow, totally posttranslational export mode exhibited by certain mutant RBP and MBP-RBP species in SecB+ cells was not affected by the loss of SecB. In contrast, MBP and RBP-MBP species with similarly altered signal peptides were totally export defective in SecB- cells. Both export-defective MBP and RBP-MBP interfered with SecB-mediated protein export by depleting cells of functional SecB. In contrast, neither export-defective RBP nor MBP-RBP elicited such an interference effect. These and other data indicated that SecB is unable to interact with precursor RBP or that any interaction between these two proteins is considerably weaker than that of SecB with precursor MBP. In addition, no correlation could be established between a SecB requirement for export and PrlA-mediated suppression of signal peptide export defects. Finally, previous studies have established that wild-type MBP export can be accomplished cotranslationally, whereas wild-type RBP export is strictly a posttranslational process. In this study, cotranslational export was not detected for either MBP-RBP or RBP-MBP. This indicates that the export mode exhibited by a given precursor protein (cotranslational versus posttranslational) is determined by properties of both the signal peptide and the mature moiety.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bankaitis V. A., Bassford P. J., Jr Proper interaction between at least two components is required for efficient export of proteins to the Escherichia coli cell envelope. J Bacteriol. 1985 Jan;161(1):169–178. doi: 10.1128/jb.161.1.169-178.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankaitis V. A., Rasmussen B. A., Bassford P. J., Jr Intragenic suppressor mutations that restore export of maltose binding protein with a truncated signal peptide. Cell. 1984 May;37(1):243–252. doi: 10.1016/0092-8674(84)90320-9. [DOI] [PubMed] [Google Scholar]
- Bankier A. T., Weston K. M., Barrell B. G. Random cloning and sequencing by the M13/dideoxynucleotide chain termination method. Methods Enzymol. 1987;155:51–93. doi: 10.1016/0076-6879(87)55009-1. [DOI] [PubMed] [Google Scholar]
- Bassford P. J., Jr, Emr S. D., Silhavy T. J., Beckwith J., Beduelle H., Clément J. M., Hedgpeth J., Hofnung M. The genetics of protein secretion in Escherichia coli. Methods Cell Biol. 1981;23:27–38. doi: 10.1016/s0091-679x(08)61489-2. [DOI] [PubMed] [Google Scholar]
- Bassford P. J., Jr Export of the periplasmic maltose-binding protein of Escherichia coli. J Bioenerg Biomembr. 1990 Jun;22(3):401–439. doi: 10.1007/BF00763175. [DOI] [PubMed] [Google Scholar]
- Bedouelle H., Bassford P. J., Jr, Fowler A. V., Zabin I., Beckwith J., Hofnung M. Mutations which alter the function of the signal sequence of the maltose binding protein of Escherichia coli. Nature. 1980 May 8;285(5760):78–81. doi: 10.1038/285078a0. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Collier D. N., Bankaitis V. A., Weiss J. B., Bassford P. J., Jr The antifolding activity of SecB promotes the export of the E. coli maltose-binding protein. Cell. 1988 Apr 22;53(2):273–283. doi: 10.1016/0092-8674(88)90389-3. [DOI] [PubMed] [Google Scholar]
- Collier D. N., Bassford P. J., Jr Mutations that improve export of maltose-binding protein in SecB- cells of Escherichia coli. J Bacteriol. 1989 Sep;171(9):4640–4647. doi: 10.1128/jb.171.9.4640-4647.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duplay P., Bedouelle H., Fowler A., Zabin I., Saurin W., Hofnung M. Sequences of the malE gene and of its product, the maltose-binding protein of Escherichia coli K12. J Biol Chem. 1984 Aug 25;259(16):10606–10613. [PubMed] [Google Scholar]
- Emr S. D., Bassford P. J., Jr Localization and processing of outer membrane and periplasmic proteins in Escherichia coli strains harboring export-specific suppressor mutations. J Biol Chem. 1982 May 25;257(10):5852–5860. [PubMed] [Google Scholar]
- Ferenci T., Silhavy T. J. Sequence information required for protein translocation from the cytoplasm. J Bacteriol. 1987 Dec;169(12):5339–5342. doi: 10.1128/jb.169.12.5339-5342.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikes J. D., Bankaitis V. A., Ryan J. P., Bassford P. J., Jr Mutational alterations affecting the export competence of a truncated but fully functional maltose-binding protein signal peptide. J Bacteriol. 1987 Jun;169(6):2345–2351. doi: 10.1128/jb.169.6.2345-2351.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikes J. D., Bassford P. J., Jr Export of unprocessed precursor maltose-binding protein to the periplasm of Escherichia coli cells. J Bacteriol. 1987 Jun;169(6):2352–2359. doi: 10.1128/jb.169.6.2352-2359.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon P. M., Li P., Kumamoto C. A. The mature portion of Escherichia coli maltose-binding protein (MBP) determines the dependence of MBP on SecB for export. J Bacteriol. 1989 Feb;171(2):813–818. doi: 10.1128/jb.171.2.813-818.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J., Lehnhardt S., Inouye M. Enhancement of protein translocation across the membrane by specific mutations in the hydrophobic region of the signal peptide. J Bacteriol. 1990 Mar;172(3):1225–1231. doi: 10.1128/jb.172.3.1225-1231.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groarke J. M., Mahoney W. C., Hope J. N., Furlong C. E., Robb F. T., Zalkin H., Hermodson M. A. The amino acid sequence of D-ribose-binding protein from Escherichia coli K12. J Biol Chem. 1983 Nov 10;258(21):12952–12956. [PubMed] [Google Scholar]
- Hutchison C. A., 3rd, Nordeen S. K., Vogt K., Edgell M. H. A complete library of point substitution mutations in the glucocorticoid response element of mouse mammary tumor virus. Proc Natl Acad Sci U S A. 1986 Feb;83(3):710–714. doi: 10.1073/pnas.83.3.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida A., Groarke J. M., Park S., Thom J., Zabicky J. H., Hazelbauer G. L., Randall L. L. A signal sequence mutant defective in export of ribose-binding protein and a corresponding pseudorevertant isolated without imposed selection. EMBO J. 1985 Jul;4(7):1875–1880. doi: 10.1002/j.1460-2075.1985.tb03863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson L. G., Randall L. L. Analysis of cotranslational proteolytic processing of nascent chains using two-dimensional gel electrophoresis. Methods Enzymol. 1983;97:77–85. doi: 10.1016/0076-6879(83)97121-5. [DOI] [PubMed] [Google Scholar]
- Josefsson L. G., Randall L. L. Different exported proteins in E. coli show differences in the temporal mode of processing in vivo. Cell. 1981 Jul;25(1):151–157. doi: 10.1016/0092-8674(81)90239-7. [DOI] [PubMed] [Google Scholar]
- Josefsson L. G., Randall L. L. Processing in vivo of precursor maltose-binding protein in Escherichia coli occurs post-translationally as well as co-translationally. J Biol Chem. 1981 Mar 10;256(5):2504–2507. [PubMed] [Google Scholar]
- Kumamoto C. A., Beckwith J. Evidence for specificity at an early step in protein export in Escherichia coli. J Bacteriol. 1985 Jul;163(1):267–274. doi: 10.1128/jb.163.1.267-274.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto C. A., Beckwith J. Mutations in a new gene, secB, cause defective protein localization in Escherichia coli. J Bacteriol. 1983 Apr;154(1):253–260. doi: 10.1128/jb.154.1.253-260.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto C. A., Chen L., Fandl J., Tai P. C. Purification of the Escherichia coli secB gene product and demonstration of its activity in an in vitro protein translocation system. J Biol Chem. 1989 Feb 5;264(4):2242–2249. [PubMed] [Google Scholar]
- Kumamoto C. A. Escherichia coli SecB protein associates with exported protein precursors in vivo. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5320–5324. doi: 10.1073/pnas.86.14.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto C. A., Gannon P. M. Effects of Escherichia coli secB mutations on pre-maltose binding protein conformation and export kinetics. J Biol Chem. 1988 Aug 15;263(23):11554–11558. [PubMed] [Google Scholar]
- Kumamoto C. A., Nault A. K. Characterization of the Escherichia coli protein-export gene secB. Gene. 1989 Jan 30;75(1):167–175. doi: 10.1016/0378-1119(89)90393-4. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Kusukawa N., Yura T., Ueguchi C., Akiyama Y., Ito K. Effects of mutations in heat-shock genes groES and groEL on protein export in Escherichia coli. EMBO J. 1989 Nov;8(11):3517–3521. doi: 10.1002/j.1460-2075.1989.tb08517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer G., Pastrana R., Sherley J., Ptashne M. Construction of overproducers of the bacteriophage 434 repressor and cro proteins. J Mol Appl Genet. 1981;1(2):139–147. [PubMed] [Google Scholar]
- Lecker S., Lill R., Ziegelhoffer T., Georgopoulos C., Bassford P. J., Jr, Kumamoto C. A., Wickner W. Three pure chaperone proteins of Escherichia coli--SecB, trigger factor and GroEL--form soluble complexes with precursor proteins in vitro. EMBO J. 1989 Sep;8(9):2703–2709. doi: 10.1002/j.1460-2075.1989.tb08411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Topping T. B., Randall L. L. Physiological role during export for the retardation of folding by the leader peptide of maltose-binding protein. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9213–9217. doi: 10.1073/pnas.86.23.9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopilato J. E., Garwin J. L., Emr S. D., Silhavy T. J., Beckwith J. R. D-ribose metabolism in Escherichia coli K-12: genetics, regulation, and transport. J Bacteriol. 1984 May;158(2):665–673. doi: 10.1128/jb.158.2.665-673.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoil C., Mekalanos J. J., Beckwith J. Alkaline phosphatase fusions: sensors of subcellular location. J Bacteriol. 1990 Feb;172(2):515–518. doi: 10.1128/jb.172.2.515-518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis S., Hunt J. F., Beckwith J. Effects of signal sequence mutations on the kinetics of alkaline phosphatase export to the periplasm in Escherichia coli. J Bacteriol. 1986 Jul;167(1):160–167. doi: 10.1128/jb.167.1.160-167.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall L. L., Hardy S. J., Thom J. R. Export of protein: a biochemical view. Annu Rev Microbiol. 1987;41:507–541. doi: 10.1146/annurev.mi.41.100187.002451. [DOI] [PubMed] [Google Scholar]
- Randall L. L., Topping T. B., Hardy S. J. No specific recognition of leader peptide by SecB, a chaperone involved in protein export. Science. 1990 May 18;248(4957):860–863. doi: 10.1126/science.2188362. [DOI] [PubMed] [Google Scholar]
- Randall L. L. Translocation of domains of nascent periplasmic proteins across the cytoplasmic membrane is independent of elongation. Cell. 1983 May;33(1):231–240. doi: 10.1016/0092-8674(83)90352-5. [DOI] [PubMed] [Google Scholar]
- Rasmussen B. A., MacGregor C. H., Ray P. H., Bassford P. J., Jr In vivo and in vitro synthesis of Escherichia coli maltose-binding protein under regulatory control of the lacUV5 promoter-operator. J Bacteriol. 1985 Nov;164(2):665–673. doi: 10.1128/jb.164.2.665-673.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J. P., Bassford P. J., Jr Post-translational export of maltose-binding protein in Escherichia coli strains harboring malE signal sequence mutations and either prl+ or prl suppressor alleles. J Biol Chem. 1985 Nov 25;260(27):14832–14837. [PubMed] [Google Scholar]
- Trun N. J., Stader J., Lupas A., Kumamoto C., Silhavy T. J. Two cellular components, PrlA and SecB, that recognize different sequence determinants are required for efficient protein export. J Bacteriol. 1988 Dec;170(12):5928–5930. doi: 10.1128/jb.170.12.5928-5930.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner K., Schatz G. Protein translocation across membranes. Science. 1988 Sep 9;241(4871):1307–1313. doi: 10.1126/science.2842866. [DOI] [PubMed] [Google Scholar]
- Walter P., Lingappa V. R. Mechanism of protein translocation across the endoplasmic reticulum membrane. Annu Rev Cell Biol. 1986;2:499–516. doi: 10.1146/annurev.cb.02.110186.002435. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Blobel G. Cytosolic factor purified from Escherichia coli is necessary and sufficient for the export of a preprotein and is a homotetramer of SecB. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2728–2732. doi: 10.1073/pnas.86.8.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Blobel G. SecB functions as a cytosolic signal recognition factor for protein export in E. coli. Cell. 1989 Aug 25;58(4):695–705. doi: 10.1016/0092-8674(89)90104-9. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Hayashi S., Wu H. C. Synthesis and export of the outer membrane lipoprotein in Escherichia coli mutants defective in generalized protein export. J Bacteriol. 1988 Sep;170(9):4001–4007. doi: 10.1128/jb.170.9.4001-4007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J. B., Bassford P. J., Jr The folding properties of the Escherichia coli maltose-binding protein influence its interaction with SecB in vitro. J Bacteriol. 1990 Jun;172(6):3023–3029. doi: 10.1128/jb.172.6.3023-3029.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J. B., MacGregor C. H., Collier D. N., Fikes J. D., Ray P. H., Bassford P. J., Jr Factors influencing the in vitro translocation of the Escherichia coli maltose-binding protein. J Biol Chem. 1989 Feb 15;264(5):3021–3027. [PubMed] [Google Scholar]
- Weiss J. B., Ray P. H., Bassford P. J., Jr Purified secB protein of Escherichia coli retards folding and promotes membrane translocation of the maltose-binding protein in vitro. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8978–8982. doi: 10.1073/pnas.85.23.8978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagursky R. J., Berman M. L. Cloning vectors that yield high levels of single-stranded DNA for rapid DNA sequencing. Gene. 1984 Feb;27(2):183–191. doi: 10.1016/0378-1119(84)90139-2. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]