Abstract
Modulation of the cholinergic neurotransmitter system results in changes in memory performance, including working memory (WM), in animals and in patients with Alzheimer disease. To identify associated changes in the functional brain response, we studied performance measures and regional cerebral blood flow (rCBF) using positron emission tomography (PET) in healthy subjects during performance of a WM task. Eight control subjects received an infusion of saline throughout the study and 13 experimental subjects received a saline infusion for the first 2 scans followed by a continuous infusion of physostigmine, an acetylcholinesterase inhibitor, for the subsequent 8 scans. rCBF was measured using H215O and PET in a sequence of 10 PET scans that alternated between rest and task scans. During task scans, subjects performed the WM task for faces. Physostigmine both improved WM efficiency, as indicated by faster reaction times, and reduced WM task-related activity in anterior and posterior regions of right midfrontal gyrus, a region shown previously to be associated with WM. Furthermore, the magnitudes of physostigmine-induced change in reaction time and right midfrontal rCBF correlated. These results suggest that enhancement of cholinergic function can improve processing efficiency and thus reduce the effort required to perform a WM task, and that activation of right prefrontal cortex is associated with task effort.
Keywords: physostigmine, acetylcholine, positron emission tomography, prefrontal cortex
Working memory (WM) refers to processes that maintain temporary, active representations of information so that they are available for further processing or recall (1, 2). The cholinergic neurotransmitter system is associated with WM, insofar as cholinergic agonists improve performance (3–5) and antagonists impair performance (6, 7) on WM tasks. Physostigmine, an acetylcholinesterase inhibitor that increases the duration of action of acetylcholine at the synapse, also improves performance on WM tasks in animals and in patients with Alzheimer disease (3–5, 8, 9).
Functional brain imaging studies of humans have identified brain regions involved in the performance of object and spatial WM tasks, including occipital, temporal, parietal, and prefrontal cortical areas (10–13). On object WM tasks, functional imaging studies in humans, as well as lesion and single neuron recording studies in nonhuman primates, indicate that posterior occipitotemporal cortex is associated more with perceptual processing, whereas prefrontal cortex is associated more with maintaining an active representation of the stimulus after it has been removed from view (10, 14–17). Although it has been shown that cholinergic modulation alters WM performance, the site of modulation in the distributed neural system that mediates WM has yet to be determined. Therefore, we decided to investigate changes in regional cerebral blood flow (rCBF) and behavioral performance (reaction time) associated with cholinergic stimulation during a WM task.
MATERIALS AND METHODS
Twenty-one right-handed healthy volunteers participated in the study. Each participant gave written informed consent after the purpose of the study and risks involved were explained (as approved by National Institute on Aging/Institutional Review Board). The control and experimental groups did not differ in mean age, years of education, or gender distribution (Table 1).
Table 1.
Subject demographics
| Control (n = 8) | Physostigmine*(n = 13) | |
|---|---|---|
| Age (yr ± SD) | 37 ± 15 | 48 ± 20 |
| Education (yr ± SD) | 16 ± 3 | 17 ± 2 |
| Gender | 6F/2M | 5F/8M |
Groups did not differ significantly.
rCBF was measured using H215O and positron emission tomography (PET) in a sequence of 10 PET scans that alternated between rest and task scans. Subjects were asked to remain awake during rest scans with their eyes open and ears unplugged. During task scans, subjects performed the WM task for faces (10). Stimuli were presented in three squares of equal size, one centered above two positioned side by side. Each item began with a 4 sec presentation of a face to remember in the upper square; followed by a 6 sec delay consisting of a 1 sec interstimulus interval, a 4 sec presentation of the three square stimulus array each containing a gray square, and another 1 sec interstimulus interval. After the delay, two test faces were presented for 4 sec, one in each of the lower two squares, and subjects indicated which test face matched the face presented at the beginning of the item by pressing a button with the right or left thumb. Faces used were novel for each trial.
Control subjects received an infusion of saline throughout the study. Experimental subjects received saline infusion for the first two scans; a loading dose of physostigmine was administered before the third scan at a rate of 1.93 mg/hr for 10 min, followed by a maintenance solution that continued to completion of the study at the rate of 0.816 mg/hr, for a total dose of 1 mg/hr of physostigmine. Blood samples were taken after each scan to assess physostigmine levels. High performance liquid chromatography assay of plasma physostigmine concentration was performed. Heart rate and blood pressure were monitored continuously during drug infusion. To reduce the potential of side effects, 0.2 mg of the peripheral cholinergic antagonist glycopyrrolate was administered (18, 19).
Using statistical parametric mapping (20) PET data were registered to correct between scan movement, spatially normalized to the orientation, size, and shape of the Talairach and Tournoux (21) atlas brain, and smoothed with a 20 mm × 20 mm × 12 mm Gaussian filter. Within- and between- group comparisons were performed on rCBF data that were adjusted with analysis of covariance to correct for differences in whole brain CBF. Statistical significance (P < 0.05) was determined based on the spatial extent of contiguous volumes of rCBF change (22). Local maxima within areas of significant change were defined as voxels with Z scores that exceeded the values for all voxels within a 18 mm × 18 mm × 20 mm volume centered on that voxel (23). Data from scans 3 through 10 were analyzed separately for both groups to identify the brain regions that were activated during task performance off and on drug, and to identify the brain regions that demonstrated drug-related alterations of activation, as demonstrated by an interaction between drug and task conditions. Within-group changes in task-related rCBF increases, comparing data from the first two scans to data from the subsequent eight scans in the drug group, were analyzed to corroborate findings from the between group analysis of the effect of physostigmine.
RESULTS
During PET studies with drug, the mean plasma concentration of physostigmine remained relatively stable with a slight but significant increase over time [F(7,84) = 9.89, P < 0.0001]. All mean physostigmine concentrations ranged between 2.2 and 2.9 ng/ml; the mean increase in plasma concentration was 0.67 ng/ml.
Reaction time (RT) improved progressively across scans during physostigmine infusion in experimental subjects [F(4,48) = 6.06, P < 0.0005], whereas RT did not change over time for control subjects (F < 1). Groups did not differ in RT before drug infusion (F < 1). Accuracy was above 90% on all task scans in both groups.
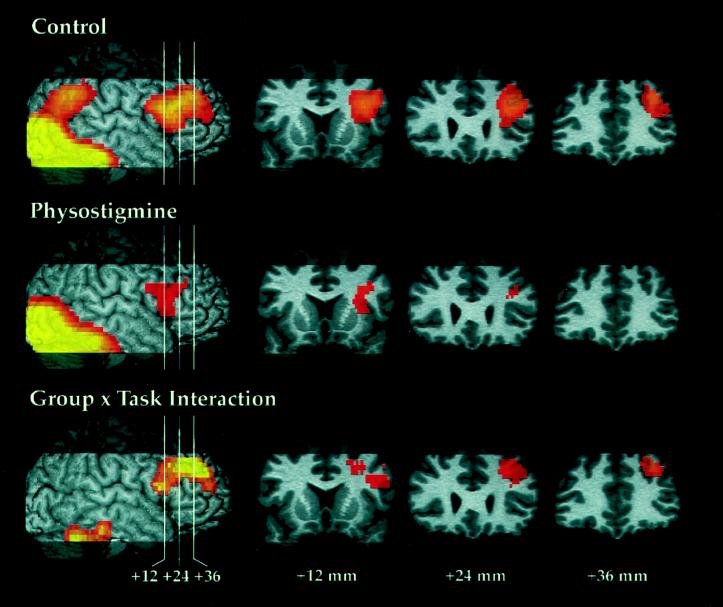
Patterns of rCBF increase during the WM task as compared with rest, on scans 3–10, are shown in Fig. 1 for the off and on drug conditions, as well as the group comparison of activations.
Figure 1.
For each comparison, the figure shows the result projected onto the right hemisphere and indicates the location for the three coronal sections in frontal cortex, located +12, +24, and +36 mm anterior to the anterior commissure. Z scores that exceeded a threshold of 2.33 (P = 0.01, two-tailed, before correction for multiple comparisons based on spatial extent) and obtained significance after an analysis of spatial extent are shown. Significant rCBF increases during WM task performance relative to rest for scans 3–10 are shown for the control and physostigmine groups. The group x task interaction shows greater task-related rCBF increases during saline infusion as compared with physostigmine infusion on comparable scans.
For control subjects, rCBF increases were observed in a large region of primary visual and visual extrastriate cortex extending from the medial occipital lobe into bilateral ventral occipitotemporal and right parietal cortex and a region of right prefrontal cortex, including posterior, inferior, and midfrontal regions. Two areas of significant rCBF increase during WM task performance relative to rest for scans 3–10 were found (Fig. 1, Control): bilateral posterior cortex (volume = 159.9 cm3, P < 0.001) with local maxima in primary visual cortex [Brodmann Area (BA) 17; 6, −102, 0], bilateral ventral occipitotemporal cortex (BA 19; 32, −64, −20; −36, −72, −20), left medial occipital cortex (BA 18; −4, −90, −12), and right parietal cortex (BA 7; 26, −64, 36); and right prefrontal cortex (volume = 23.3 cm3, P < 0.001) with local maxima in the posterior midfrontal gyrus (BA 45; 38, 20, 24) and anterior midfrontal gyrus (BA 9; 34, 34, 36). The right prefrontal activation extended into the inferior frontal gyrus, but no local maxima were found there.
Performance of the WM task during physostigmine infusion similarly was associated with activation in posterior cortical areas, and with a smaller activation in right prefrontal cortex. In the experimental group, two areas of significant rCBF increase during WM task performance relative to rest on scans 3–10 were found (Fig. 1, Physostigmine): bilateral posterior cortex (volume = 149.7 cm3, P < 0.001) with local maxima in primary visual cortex (BA 17; −6, −100, −12; 12, −92, −8); and right prefrontal cortex (volume = 4.4 cm3, P < 0.05) with local maxima in the posterior midfrontal gyrus (BA 45; 32, 12, 20) and inferior frontal gyrus (BA 44; 32, 12, 4). Unlike the control subject results, the activation did not extend into the anterior midfrontal gyrus. A right parietal area was seen in the experimental group, corresponding to that seen in the control group, but was not of sufficient spatial extent to achieve statistical significance based on our criteria.
The group x task interaction (Fig. 1), showing greater task-related rCBF increases during saline infusion as compared with physostigmine infusion on comparable scans, indicated that the right ventral region of inferior temporal/cerebellar cortex (volume = 6.1 cm3; P < 0.01; local maxima: 44, −60, −24; 26, −50, −28; 20, −42, −20), as well as a large area of right prefrontal cortex (volume = 12.6 cm3, P < 0.0001) with local maxima both in posterior (BA 44; 48, 8, 16; 26, 16, 36; 48, 18, 32) and anterior (BA 9; 28, 38, 36; 36, 50, 20) regions in the midfrontal gyrus, were less activated during physostigmine infusion as compared with saline infusion. No significant region with smaller rCBF increases on saline infusion was found.
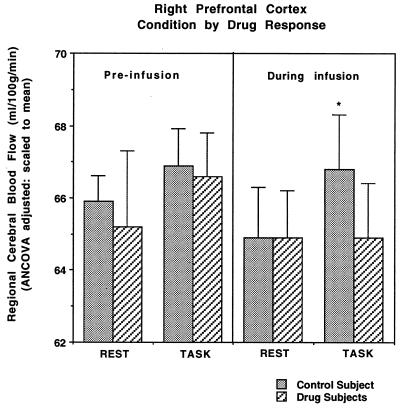
Mean rCBF values for the right prefrontal region in the midfrontal gyrus (BA 9/44), which demonstrated a significant group x task interaction are presented in Fig. 2 and show that infusion of physostigmine alters rCBF in this region only when it is participating in WM performance and not at rest [F(3,202)= 6.62, P < 0.0003]. Prior to infusion, rCBF values for control subjects and experimental subjects did not differ significantly, and both groups showed similar task-related rCBF increases (P > 0.1 for main effects of group and task and for their interaction). During the WM task, the control subjects showed a rCBF response that was significantly higher than that seen in the drug group [F(1,57) = 64.5; P < 0.0001]. Subjects in the physostigmine group showed no change in rCBF from rest to task in this same right midfrontal region (P > 0.1), which showed almost no overlap with the right inferior frontal region that was activated. No difference was observed in resting state rCBF [P > 0.1 for main effects of group (drug vs. control) and time (pre- vs. post-infusion) and for their interaction].
Figure 2.
The group x task interaction for mean rCBF values from the right prefrontal region that showed a significant group difference in the size of task-related rCBF increases (Fig. 1), is shown.
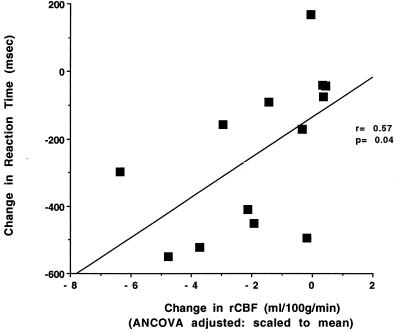
A direct, within-group comparison of rCBF during the WM task on drug (scans 3–10) to rCBF during the WM task off drug (scans 1 and 2) in the experimental subjects confirmed the difference seen between the experimental and control groups in the right prefrontal region but not in the right anterior extrastriate region. Task-related right prefrontal rCBF increases were significantly reduced during physostigmine administration in a region that included the anterior midfrontal gyrus as well as inferior frontal and anterior cingulate cortex. Moreover, the magnitude of the reduction correlated significantly with the decrease in RT (r = 0.57, P < 0.05) during physostigmine infusion (Fig. 3), indicating that greater improvements in RT were associated with larger reductions in right prefrontal rCBF. The magnitudes represent differences between the RT and rCBF measures (using the local maximum from the within-group analysis of drug effect on task; 20, 20, 28) during the first WM task PET scan, obtained in the absence of drug, and the final WM task PET scan, obtained during physostigmine infusion. Because no region in right extrastriate cortex was seen that demonstrated within-group changes in task-related rCBF increases corresponding to that seen in the group by task interaction, this remains a suggestive but less robust finding. No significant change in rCBF was observed in the control group (no drug) with comparable subtractions.
Figure 3.
A significant correlation was found between the magnitudes of the physostigmine-induced changes in RT and rCBF in right prefrontal cortex [F(1,11) = 5.3, P < 0.05], indicating that greater improvements in RT were associated with larger reductions in right prefrontal rCBF.
DISCUSSION
The results of this study show that enhancement of cholinergic neurotransmission results in an improvement in WM efficiency that is correlated with an alteration of brain activity in a cortical region known to play a central role in WM. Moreover, this local alteration in brain activity is evident only during performance of the WM task and not at rest. Cholinergic agents affect memory function in animals and humans. For example, tacrine, a long-acting acetylcholinesterase inhibitor, is used clinically to treat cognitive symptoms of Alzheimer disease. The brain mechanism by which tacrine improves cognitive performance is unknown. For the first time, however, we have demonstrated a cholinergically induced modulation of functional brain response to a memory task that was related to the observed improvement in behavior.
Previous work also has shown that cholinergic enhancement using physostigmine does not affect resting rCBF in healthy human subjects (24), although in patients with Alzheimer disease, resting rCBF in hypometabolic cortical regions is increased by physostigmine (24, 25). In a previous PET study, the dopaminergic agonist, apomorphine, was found to impair memory performance and diminish memory task-related rCBF increases in prefrontal cortex (26).
Given that acetylcholinesterase inhibitors prolong acetylcholine activity at the synapse, one might expect improvement in performance to be associated with increased rCBF in a task-specific brain region, yet we observed a reduction in right prefrontal rCBF. One possible explanation is that reduced activation in right prefrontal cortex may reflect the shorter time required to perform the WM task, although several papers, including a recent functional magnetic resonance imaging study, indicate that right prefrontal activity is associated more with maintenance of an active representation over the memory delay than with response selection (10, 14–17).
The observed reduction of right prefrontal activity might have been a direct effect of physostigmine. Anatomically, the synaptic circuitry of cholinergic prefrontal fibers includes symmetric synapses on pyramidal cells (27); symmetric synaptic morphology is characteristic of inhibitory mechanisms (28–30). It is unclear, however, why a direct inhibition of right prefrontal activity would be correlated with improved WM performance. Moreover, direct inhibition demands neuronal activity and therefore may result in increased rather than decreased cerebral blood flow.
An alternative explanation for the physostigmine-induced reduction of the right prefrontal rCBF response is that right prefrontal activity is associated with the effort needed to perform the WM task, and that this region is recruited as the effort required to perform the task increases. Prefrontal cortex may become more active with tasks that require a greater allocation of attentional resources (31). A PET study of word list recall showed that increased memory load resulted in greater rCBF increases in frontal cortex as well as in other brain structures (32). Another PET study showed that increases in the difficulty of a face-matching task increased rCBF in the right midfrontal gyrus with a local maximum 6 mm from one of the anterior local maxima identified in this study (33). Similarly, studies of event-related electrophysiological potentials show that the response amplitude in the prefrontal cortex increases with task difficulty during WM (34, 35). The anticholinesterase effects of physostigmine may enhance efficiency of WM processes, thus reducing the effort required to perform the task and the need to recruit prefrontal cortex. The mechanism by which physostigmine reduces effortful processing during WM remains unclear. Physostigmine may enhance efficiency by amplifying processing of information in the focus of attention (36) or by minimizing the effects of distracting stimuli (35).
Acknowledgments
We would like to express our appreciation to Dr. Alex Martin, Dr. Leslie Ungerleider, and Dr. Dan Longo for their careful and critical review of the manuscript. This research was supported by the National Institute on Aging intramural program.
ABBREVIATIONS
- PET
positron emission tomography
- rCBF
regional cerebral blood flow
- RT
reaction time
- WM
working memory
- BA
Brodmann Area
References
- 1.Baddeley A, Logie R, Bressi S, Della Sala S, Spinnler H. Q J Exp Psychol. 1986;38:603–618. doi: 10.1080/14640748608401616. [DOI] [PubMed] [Google Scholar]
- 2.Baddeley A D, Bressi S, Della Sala S, Logie R, Spinnler H. Brain. 1991;114:2521–2542. doi: 10.1093/brain/114.6.2521. [DOI] [PubMed] [Google Scholar]
- 3.Glasky A J, Melchior C L, Pirzadeh B, Heydari N, Ritsmann R F. Pharmacol Biochem Behav. 1994;47:325–329. doi: 10.1016/0091-3057(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 4.Terry A V, Jackson W J, Buccafusco J J. Cereb Cortex. 1993;3:304–312. doi: 10.1093/cercor/3.4.304. [DOI] [PubMed] [Google Scholar]
- 5.Kitajima I, Yamamoto T, Ohno M, Ueki S. Jpn J Pharmacol. 1992;58:175–183. doi: 10.1254/jjp.58.175. [DOI] [PubMed] [Google Scholar]
- 6.Dawson G R, Iverson S D. Behav Brain Res. 1993;57:143–153. doi: 10.1016/0166-4328(93)90130-i. [DOI] [PubMed] [Google Scholar]
- 7.Rusted J M, Warburton D M. Psychopharmacology. 1988;96:145–152. doi: 10.1007/BF00177553. [DOI] [PubMed] [Google Scholar]
- 8.Levy A, Brandeis R, Treves T A, Meshulam Y, Mawassi F, Feiler D, Wengier A, Glikfeld P, Grunwald J, Dachir S, Rabey J M, Levy D, Korczyn A D. Alzheimer Dis Assoc Disord. 1994;8:15–21. doi: 10.1097/00002093-199408010-00004. [DOI] [PubMed] [Google Scholar]
- 9.Sano M, Bell K, Marder K, Stricks L, Stern Y, Mayeux R. Clin Neuropharmacol. 1993;16:61–69. doi: 10.1097/00002826-199302000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Haxby J V, Ungerleider L G, Horwitz B, Rapoport S I, Grady C L. Hum Brain Mapp. 1995;3:68–82. doi: 10.1002/(SICI)1097-0193(1996)4:4<227::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Freidman H R, Goldman-Rakic P S. J Neurosci. 1994;14:2775–2788. doi: 10.1523/JNEUROSCI.14-05-02775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrides M, Alivisatos B, Evans A C, Meyer E. Proc Natl Acad Sci USA. 1993;90:873–877. doi: 10.1073/pnas.90.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarthy G, Blamire A M, Puce A, Nobre A C, Block G, Hyder F, Goldman-Rakic P, Shulman R G. Proc Natl Acad Sci USA. 1994;91:8690–8694. doi: 10.1073/pnas.91.18.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Funahashi S, Chafee M V, Goldman-Rakic P S. Nature (London) 1993;365:753–756. doi: 10.1038/365753a0. [DOI] [PubMed] [Google Scholar]
- 15.Miller E K, Desimone R. J Neurosci. 1993;13:1460–1478. doi: 10.1523/JNEUROSCI.13-04-01460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shindy W W, Posley K A, Fuster J M. Cereb Cortex. 1994;4:443–450. doi: 10.1093/cercor/4.4.443. [DOI] [PubMed] [Google Scholar]
- 17.Courtney S M, Ungerleider L G, Keil K, Haxby J V. Nature (London) 1997;386:608–611. doi: 10.1038/386608a0. [DOI] [PubMed] [Google Scholar]
- 18.Oduro K A. Can Anaesth Soc J. 1975;22:466–473. doi: 10.1007/BF03004861. [DOI] [PubMed] [Google Scholar]
- 19.Mirakhur R K, Dundee J W, Clarke R S. Br J Anaesth. 1977;49:825–829. doi: 10.1093/bja/49.8.825. [DOI] [PubMed] [Google Scholar]
- 20.Friston K J, Holmes A P, Worsley K J, Poline J-B, Frith C D, Frackowiak R S J. Hum Brain Mapp. 1995;2:189–210. doi: 10.1002/(SICI)1097-0193(1996)4:2<140::AID-HBM5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 22.Friston K J, Worsley K J, Frackowiak R S J, Mazziotta J C, Evans A C. Hum Brain Mapp. 1994;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- 23.Haxby J V, Horwitz B, Ungerleider L G, Maisog J M, Pietrini P, Grady C L. J Neurosci. 1994;14:6336–6353. doi: 10.1523/JNEUROSCI.14-11-06336.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geaney D P, Soper N, Shepstone B J, Cowen P J. Lancet. 1990;335:1484–1487. doi: 10.1016/0140-6736(90)93028-n. [DOI] [PubMed] [Google Scholar]
- 25.Gustafson L, Edivisson L, Dahlgren N, Hagberg B, Risberg J, Rosen I, Ferno H. Psychopharmacology. 1987;93:31–35. doi: 10.1007/BF02439583. [DOI] [PubMed] [Google Scholar]
- 26.Friston K J, Grasby P M, Bench C J, Frith C D, Cowen P J, Liddle P F, Frackowiak R S J, Dolan R. Neurosci Lett. 1992;141:106–110. doi: 10.1016/0304-3940(92)90345-8. [DOI] [PubMed] [Google Scholar]
- 27.Mrzljak L, Pappy M, Leranth C, Goldman-Rakic P S. J Comp Neurol. 1995;357:603–617. doi: 10.1002/cne.903570409. [DOI] [PubMed] [Google Scholar]
- 28.Dobson V G. Perception. 1981;10:483–510. doi: 10.1068/p100483. [DOI] [PubMed] [Google Scholar]
- 29.Peters A, Proskauer C C, Ribak C E. J Comp Neurol. 1982;206:397–416. doi: 10.1002/cne.902060408. [DOI] [PubMed] [Google Scholar]
- 30.Soriano E, Martinez A, Farinas I, Frotscher M. J Comp Neurol. 1993;337:151–167. doi: 10.1002/cne.903370110. [DOI] [PubMed] [Google Scholar]
- 31.Posner M I, Petersen S E. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- 32.Grasby P M, Frith C D, Friston K J, Simpson J, Fletcher P C, Frackowiak R S J, Dolan R J. Brain. 1994;117:1271–1282. doi: 10.1093/brain/117.6.1271. [DOI] [PubMed] [Google Scholar]
- 33.Grady C L, Horwitz B, Pietrini P, Mentis M, Ungerleider L G, Rapoport S I, Haxby J V. Hum Brain Mapp. 1996;4:227–239. doi: 10.1002/(SICI)1097-0193(1996)4:4<227::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 34.Chao L L, Nielson-Bohlman L, Knight R T. Electroencephalogr Clin Neurophysiol. 1995;96:157–169. doi: 10.1016/0168-5597(94)00256-e. [DOI] [PubMed] [Google Scholar]
- 35.Chao L L, Knight R T. Cognit Brain Res. 1996;4:27–37. doi: 10.1016/0926-6410(95)00043-7. [DOI] [PubMed] [Google Scholar]
- 36.Callaway E, Halliday R, Naylor H. Biol Psychiatry. 1992;33:1–22. doi: 10.1016/0301-0511(92)90002-c. [DOI] [PubMed] [Google Scholar]