Abstract
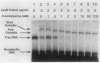
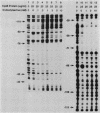
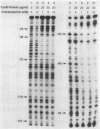
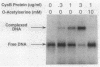
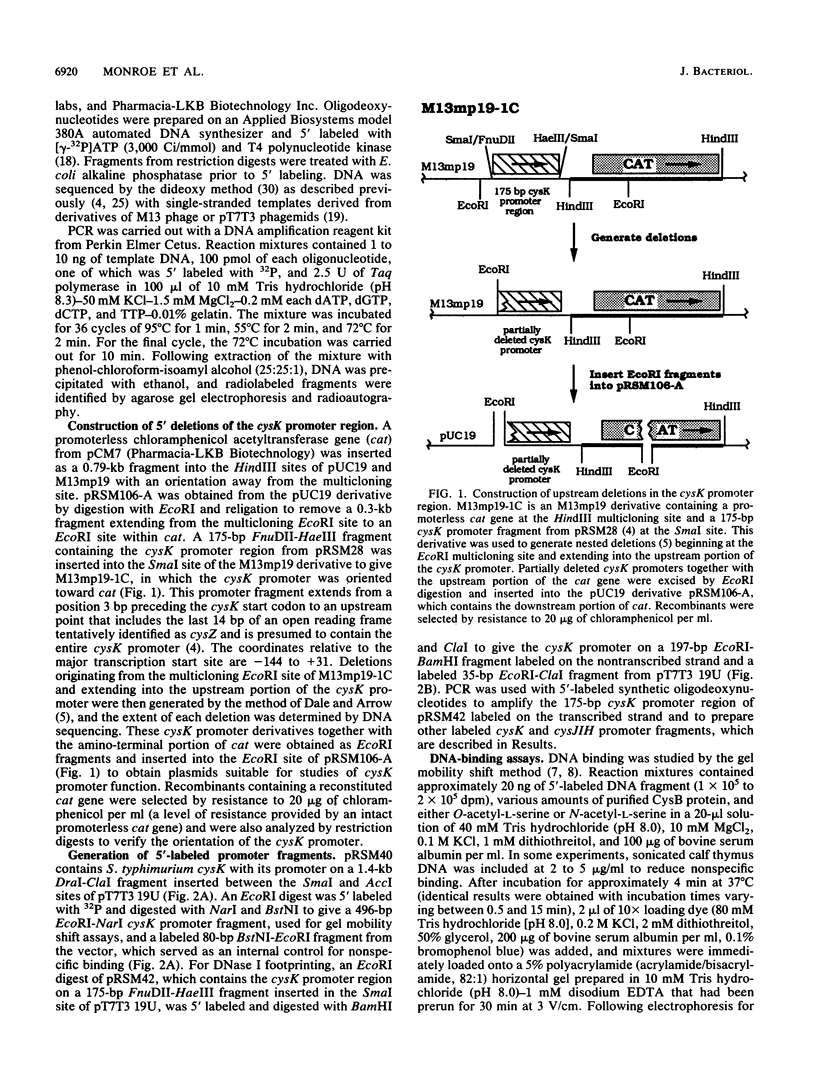
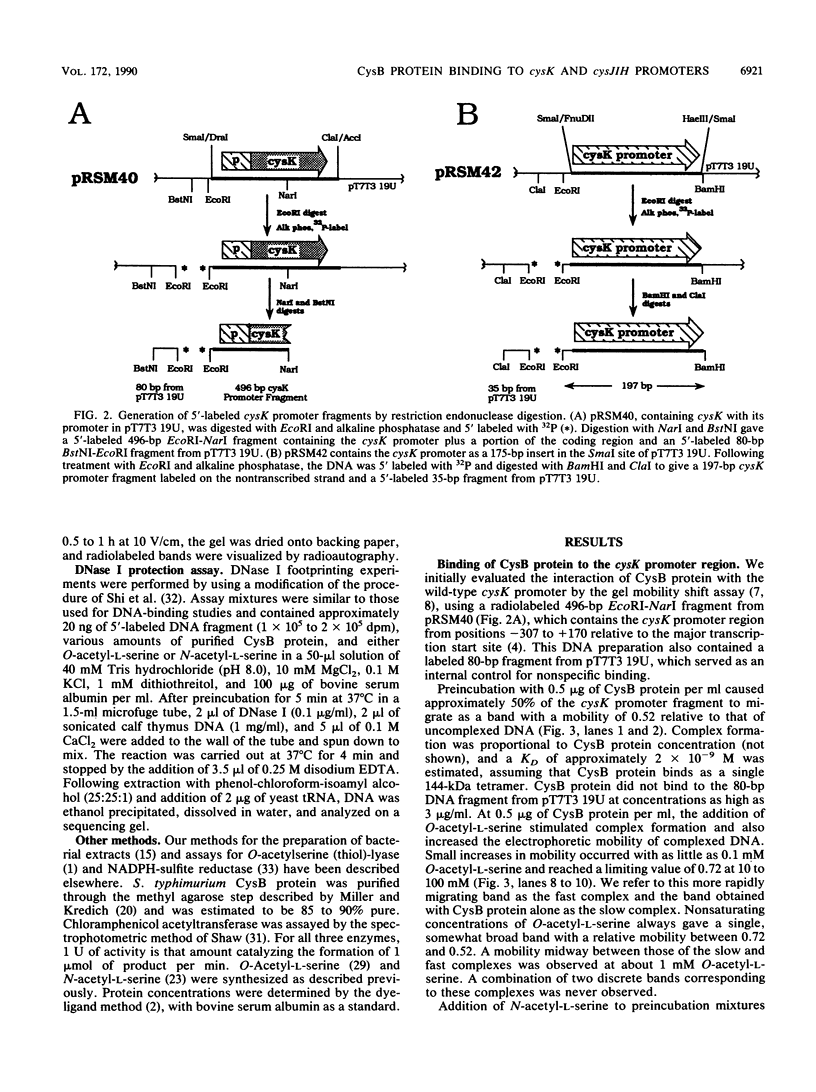
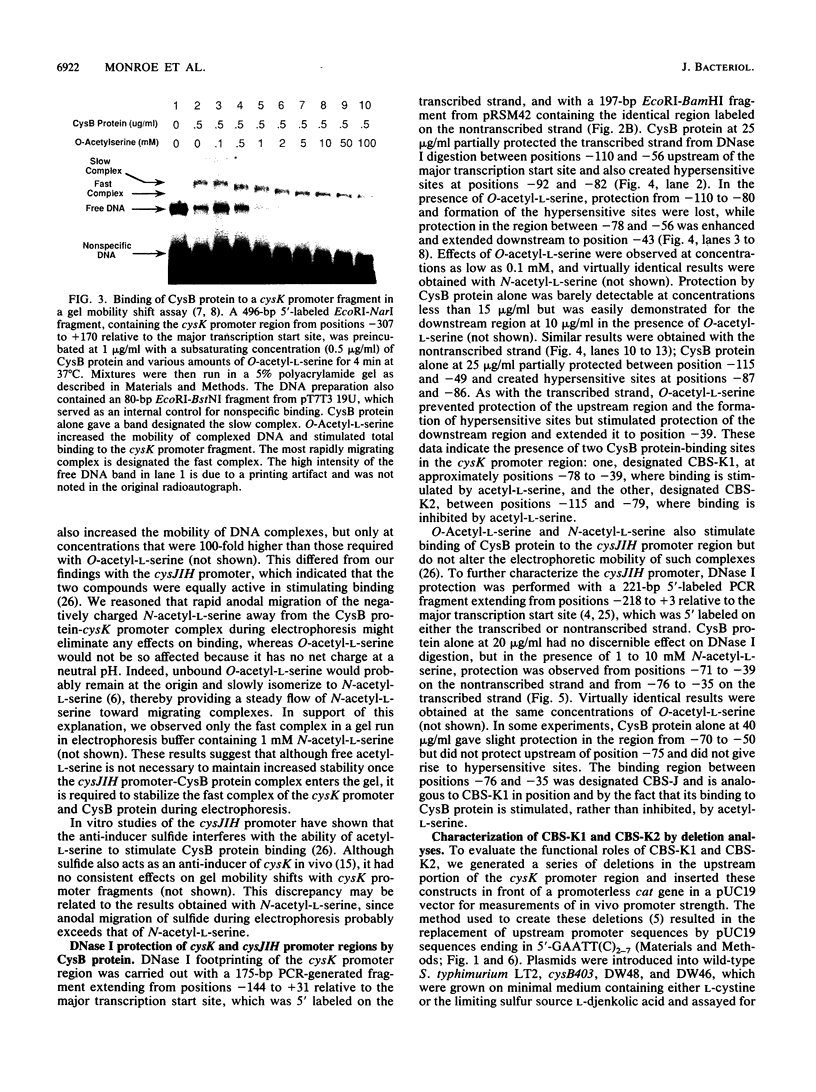
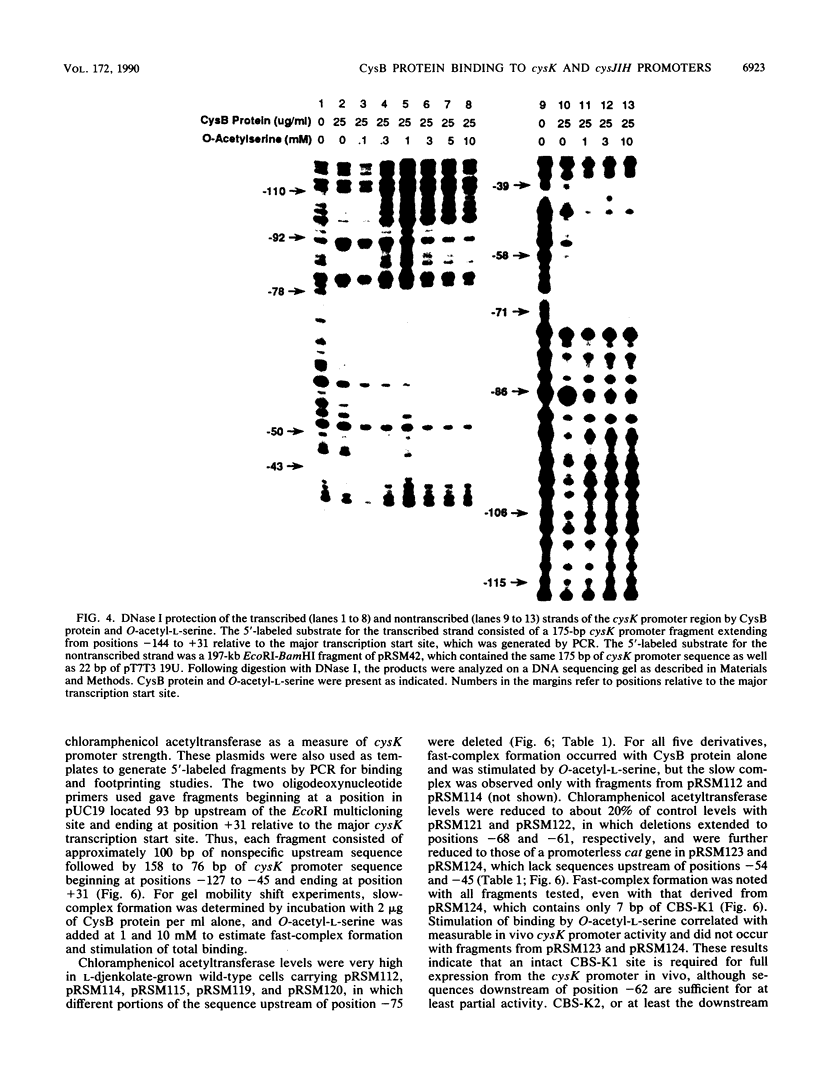
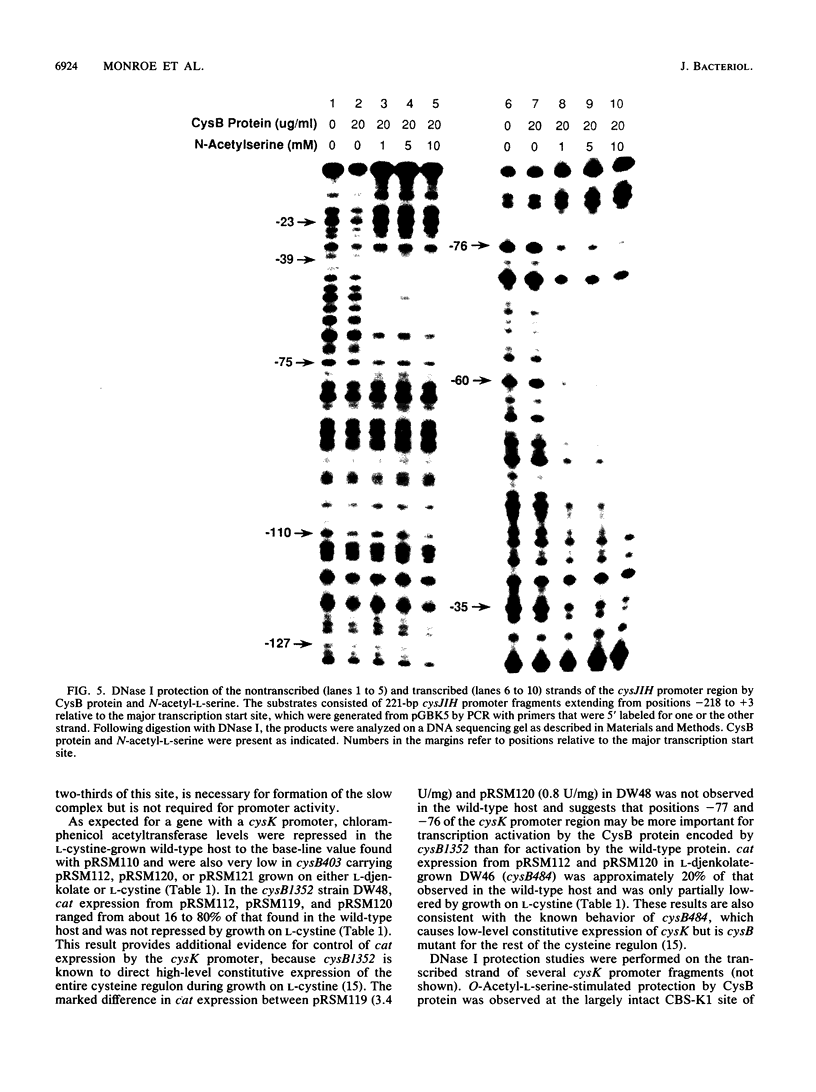
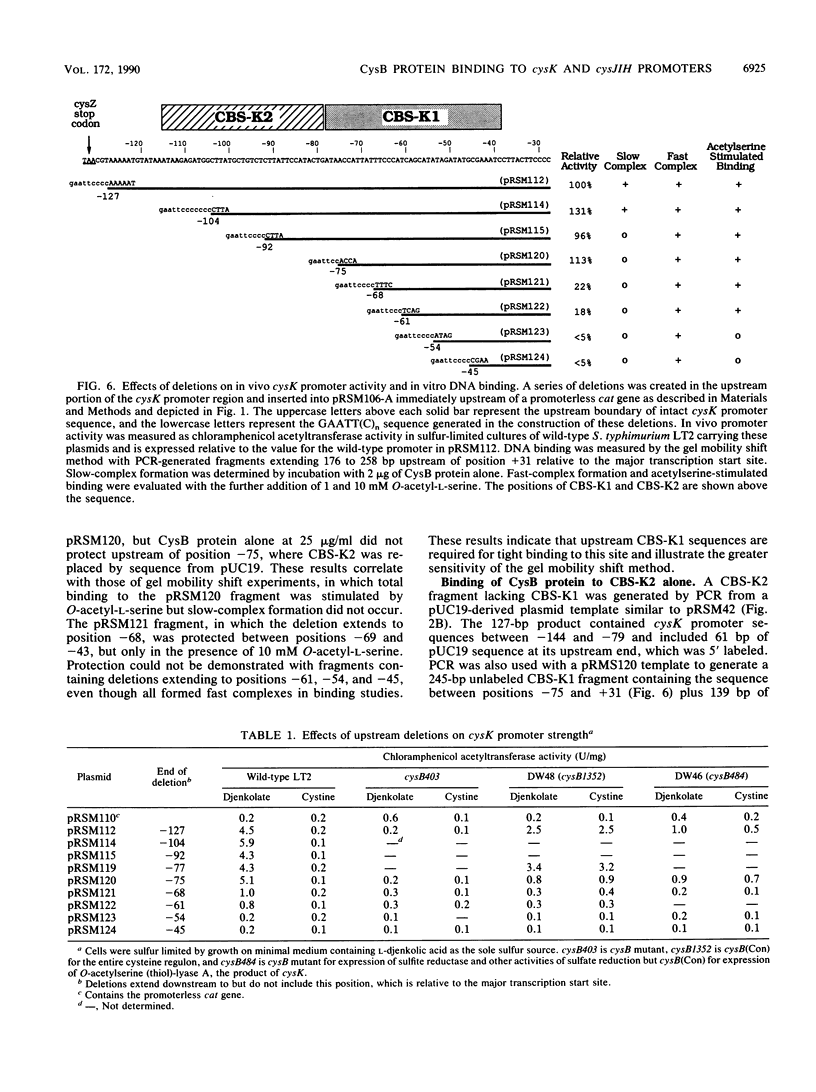
The cysteine regulons of Salmonella typhimurium and Escherichia coli are positively regulated by CysB protein and either O-acetyl-L-serine or N-acetyl-L-serine, both of which act as inducers. Gel mobility shift assays and DNase I footprinting experiments showed that CysB protein binds to the S. typhimurium cysK promoter at two sites, one, designated CBS-K1, at positions -78 to -39 relative to the major transcription start site, and the other, designated CBS-K2, at positions -115 to -79. The S. typhimurium cysJIH promoter was found to contain a single binding site, designated CBS-JH, at positions -76 to -35. Acetyl-L-serine stimulated binding to CBS-K1 and CBS-J and inhibited binding to CBS-K2. In the absence of acetyl-L-serine, CysB protein bound to both CBS-K1 and CBS-K2 and gave a complex that migrated more slowly during gel electrophoresis than did that formed in the presence of acetyl-L-serine, in which case CysB protein bound only to CBS-K1. Complexes formed with DNA containing the two binding sites either at the middle or at one end of the fragment migrated differently, suggesting that DNA was bent in the slow complex formed in the absence of acetyl-L-serine and that DNA in the fast complex was less bent or not bent at all. An analysis of upstream deletions of the cysK promoter showed that only CBS-K1 is required for in vivo promoter activity. CBS-J is analogous in position to CBS-K1 and is probably also required for activity of the cysJIH promoter. CBS-K2 has no known function but may help sequester CysB protein at the cysK promoter.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker M. A., Kredich N. M., Tomkins G. M. The purification and characterization of O-acetylserine sulfhydrylase-A from Salmonella typhimurium. J Biol Chem. 1969 May 10;244(9):2418–2427. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bullas L. R., Ryu J. I. Salmonella typhimurium LT2 strains which are r- m+ for all three chromosomally located systems of DNA restriction and modification. J Bacteriol. 1983 Oct;156(1):471–474. doi: 10.1128/jb.156.1.471-474.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne C. R., Monroe R. S., Ward K. A., Kredich N. M. DNA sequences of the cysK regions of Salmonella typhimurium and Escherichia coli and linkage of the cysK regions to ptsH. J Bacteriol. 1988 Jul;170(7):3150–3157. doi: 10.1128/jb.170.7.3150-3157.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., Arrow A. A rapid single-stranded cloning, sequencing, insertion, and deletion strategy. Methods Enzymol. 1987;155:204–214. doi: 10.1016/0076-6879(87)55017-0. [DOI] [PubMed] [Google Scholar]
- Flavin M., Slaughter C. Synthesis of the succinic ester of homoserine, a new intermediate in the bacterial biosynthesis of methionine. Biochemistry. 1965 Jul;4(7):1370–1375. doi: 10.1021/bi00883a022. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S., Haughn G. W., Calvo J. M., Wallace J. C. A large family of bacterial activator proteins. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6602–6606. doi: 10.1073/pnas.85.18.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulanicka M. D., Kredich N. M., Treiman D. M. The structural gene for O-acetylserine sulfhydrylase A in Salmonella typhimurium. Identity with the trzA locus. J Biol Chem. 1974 Feb 10;249(3):867–872. [PubMed] [Google Scholar]
- Jagura-Burdzy G., Hulanicka D. Use of gene fusions to study expression of cysB, the regulatory gene of the cysteine regulon. J Bacteriol. 1981 Sep;147(3):744–751. doi: 10.1128/jb.147.3.744-751.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Mortimer M. C. Positive control of sulphate reduction in Escherichia coli. The nature of the pleiotropic cysteineless mutants of E. coli K12. Biochem J. 1968 Dec;110(3):597–602. doi: 10.1042/bj1100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Mortimer M. C., Wheldrake J. F., Pasternak C. A. The control of sulphate reduction in Escherichia coli by O-acetyl-L-serine. Biochem J. 1968 Mar;107(1):51–53. doi: 10.1042/bj1070051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredich N. M. Regulation of L-cysteine biosynthesis in Salmonella typhimurium. I. Effects of growth of varying sulfur sources and O-acetyl-L-serine on gene expression. J Biol Chem. 1971 Jun 10;246(11):3474–3484. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Miller B. E., Kredich N. M. Purification of the cysB protein from Salmonella typhimurium. J Biol Chem. 1987 May 5;262(13):6006–6009. [PubMed] [Google Scholar]
- Monroe R. S., Kredich N. M. Isolation of Salmonella typhimurium cys genes by transduction with a library of recombinant plasmids packaged in bacteriophage P22HT capsids. J Bacteriol. 1988 Jan;170(1):42–47. doi: 10.1128/jb.170.1.42-47.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARITA K. Isolation of acetylseryltyrosine from the chymotryptic digests of proteins of five strains of tobacco mosaic virus. Biochim Biophys Acta. 1958 Nov;30(2):352–359. doi: 10.1016/0006-3002(58)90060-x. [DOI] [PubMed] [Google Scholar]
- Ostrowski J., Jagura-Burdzy G., Kredich N. M. DNA sequences of the cysB regions of Salmonella typhimurium and Escherichia coli. J Biol Chem. 1987 May 5;262(13):5999–6005. [PubMed] [Google Scholar]
- Ostrowski J., Kredich N. M. In vitro interactions of CysB protein with the cysJIH promoter of Salmonella typhimurium: inhibitory effects of sulfide. J Bacteriol. 1990 Feb;172(2):779–785. doi: 10.1128/jb.172.2.779-785.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski J., Kredich N. M. Molecular characterization of the cysJIH promoters of Salmonella typhimurium and Escherichia coli: regulation by cysB protein and N-acetyl-L-serine. J Bacteriol. 1989 Jan;171(1):130–140. doi: 10.1128/jb.171.1.130-140.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raibaud O., Schwartz M. Positive control of transcription initiation in bacteria. Annu Rev Genet. 1984;18:173–206. doi: 10.1146/annurev.ge.18.120184.001133. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Shi Y. B., Gamper H., Van Houten B., Hearst J. E. Interaction of Escherichia coli RNA polymerase with DNA in an elongation complex arrested at a specific psoralen crosslink site. J Mol Biol. 1988 Jan 20;199(2):277–293. doi: 10.1016/0022-2836(88)90314-2. [DOI] [PubMed] [Google Scholar]
- Siegel L. M., Davis P. S., Kamin H. Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of enterobacteria. 3. The Escherichia coli hemoflavoprotein: catalytic parameters and the sequence of electron flow. J Biol Chem. 1974 Mar 10;249(5):1572–1586. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wu H. M., Crothers D. M. The locus of sequence-directed and protein-induced DNA bending. Nature. 1984 Apr 5;308(5959):509–513. doi: 10.1038/308509a0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]