Abstract
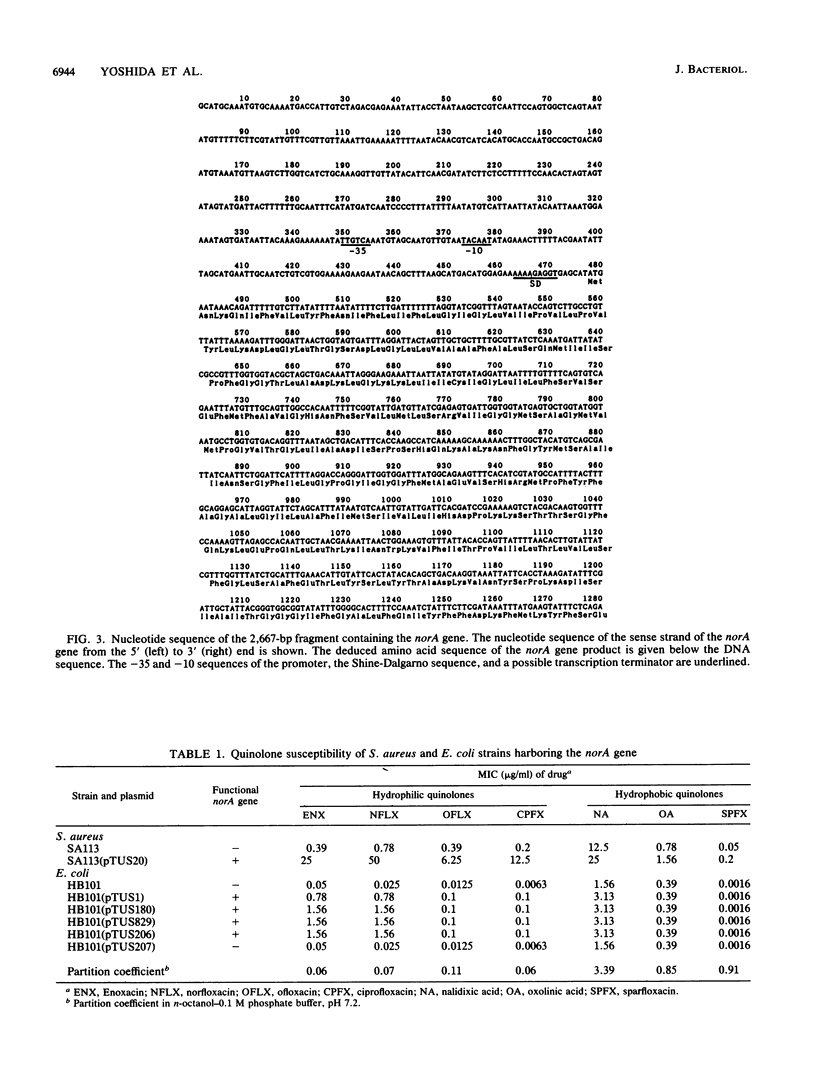
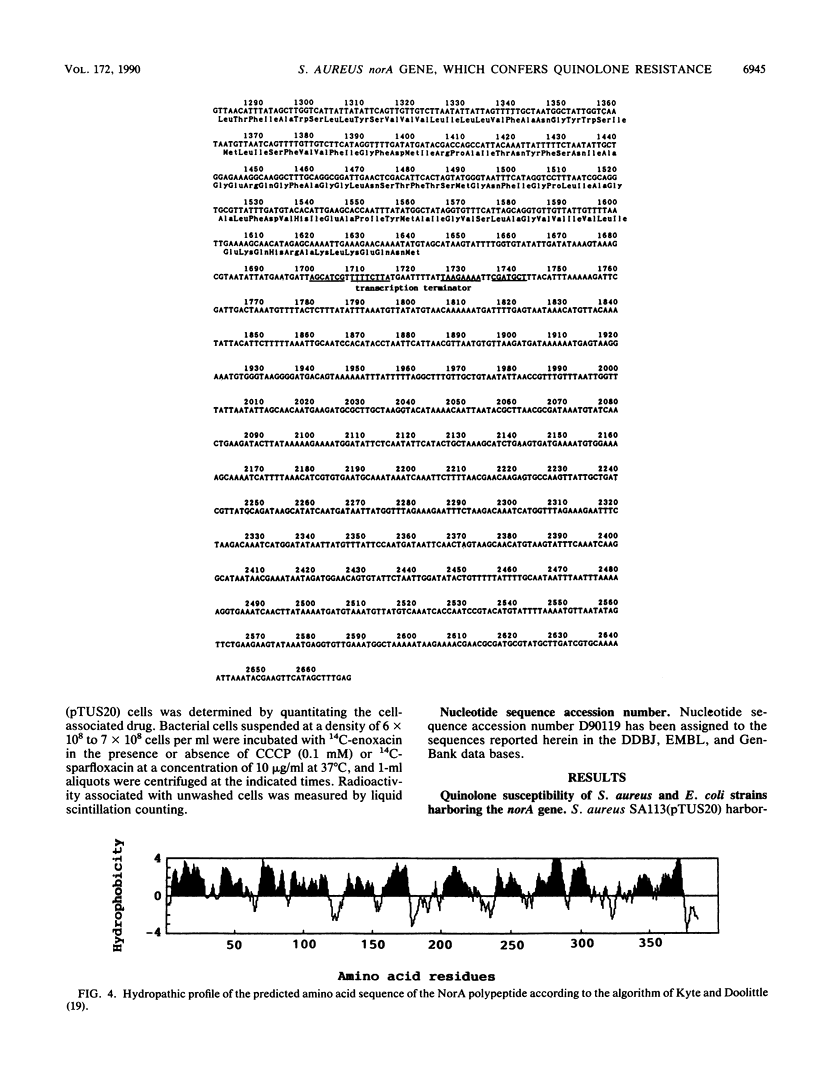
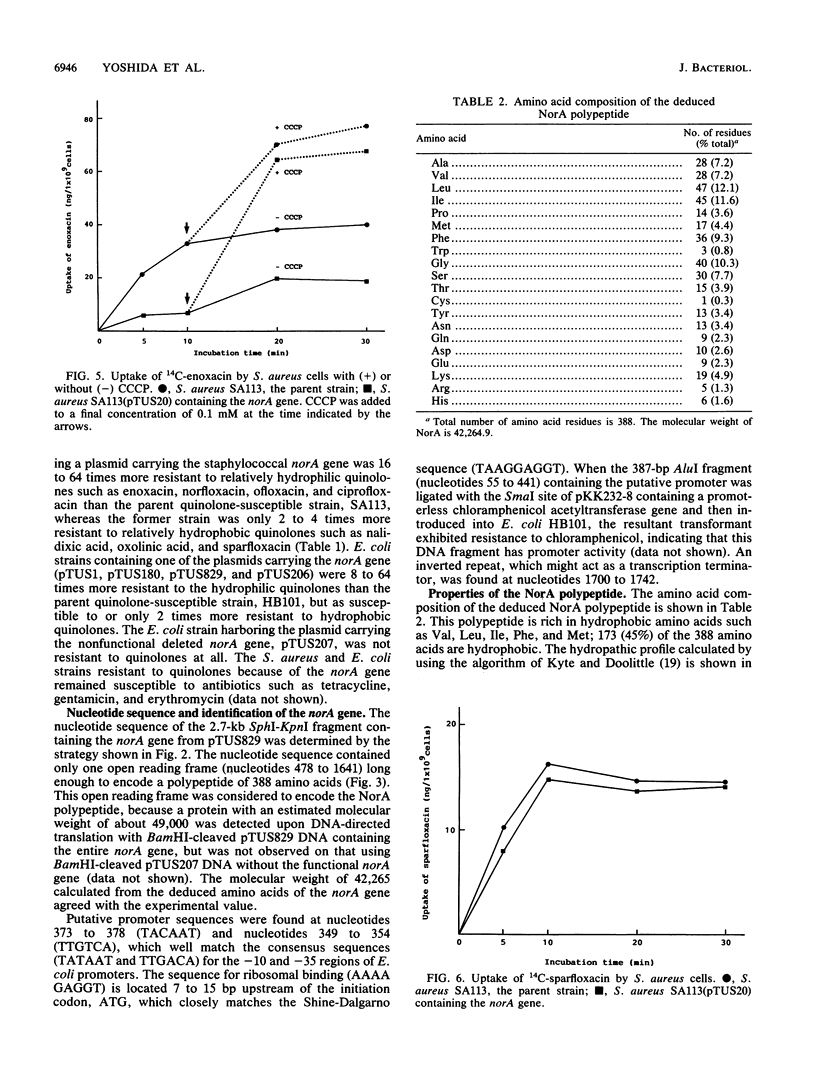
The norA gene cloned from chromosomal DNA of quinolone-resistant Staphylococcus aureus TK2566 conferred relatively high resistance to hydrophilic quinolones such as norfloxacin, enoxacin, ofloxacin, and ciprofloxacin, but only low or no resistance at all to hydrophobic ones such as nalidixic acid, oxolinic acid, and sparfloxacin in S. aureus and Escherichia coli. The 2.7-kb DNA fragment containing the norA gene had a long open reading frame coding for 388 amino acid residues with a molecular weight of 42,265, which was consistent with the experimental value of about 49,000 obtained on DNA-directed translation. The deduced NorA polypeptide has 12 hydrophobic membrane-spanning regions and is partly homologous to tetracycline resistance protein and sugar transport proteins. The uptake of a hydrophilic quinolone, enoxacin, by S. aureus harboring a plasmid carrying the norA gene was about 50% that by the parent strain lacking the plasmid, but it increased to almost the same level as that by the latter strain with carbonyl cyanide m-chlorophenyl hydrazone. On the other hand, the uptake of a hydrophobic quinolone, sparfloxacin, was similar in the two strains. These results suggest that the NorA polypeptide may constitute a membrane-associated active efflux pump of hydrophilic quinolones.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaum M. J., Haspel H. C., Rosen O. M. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon G. J., Levitt M., Sternglanz R. Studies on the mechanism of action of nalidixic acid. Antimicrob Agents Chemother. 1973 Oct;4(4):479–486. doi: 10.1128/aac.4.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl C. J., Deber C. M. Hypothesis about the function of membrane-buried proline residues in transport proteins. Proc Natl Acad Sci U S A. 1986 Feb;83(4):917–921. doi: 10.1073/pnas.83.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberland S., Bayer A. S., Schollaardt T., Wong S. A., Bryan L. E. Characterization of mechanisms of quinolone resistance in Pseudomonas aeruginosa strains isolated in vitro and in vivo during experimental endocarditis. Antimicrob Agents Chemother. 1989 May;33(5):624–634. doi: 10.1128/aac.33.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. P., Hooper D. C., Wolfson J. S., Souza K. S., McMurry L. M., Levy S. B. Endogenous active efflux of norfloxacin in susceptible Escherichia coli. Antimicrob Agents Chemother. 1988 Aug;32(8):1187–1191. doi: 10.1128/aac.32.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman D. C., Chopra I., Shales S. W., Howe T. G., Foster T. J. Analysis of tetracycline resistance encoded by transposon Tn10: deletion mapping of tetracycline-sensitive point mutations and identification of two structural genes. J Bacteriol. 1983 Feb;153(2):921–929. doi: 10.1128/jb.153.2.921-929.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Itoh T., Tomizawa J. I. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane M. W., Wood T. H. Escherichia coli K-12 mutants resistant to nalidixic acid: genetic mapping and dominance studies. J Bacteriol. 1969 Jul;99(1):238–241. doi: 10.1128/jb.99.1.238-241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa I., Hiramitsu T., Tanaka Y. Synthesis and antibacterial activities of substituted 7-oxo-2,3-dihydro-7H-pyrido[1,2,3-de][1,4]benzoxazine-6-carboxyl ic acids. Chem Pharm Bull (Tokyo) 1984 Dec;32(12):4907–4913. doi: 10.1248/cpb.32.4907. [DOI] [PubMed] [Google Scholar]
- Higgins N. P., Peebles C. L., Sugino A., Cozzarelli N. R. Purification of subunits of Escherichia coli DNA gyrase and reconstitution of enzymatic activity. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1773–1777. doi: 10.1073/pnas.75.4.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Isolation and characterization of norfloxacin-resistant mutants of Escherichia coli K-12. Antimicrob Agents Chemother. 1986 Aug;30(2):248–253. doi: 10.1128/aac.30.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Mutations producing resistance to norfloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1987 Apr;31(4):582–586. doi: 10.1128/aac.31.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Tung C., McHugh G. L., Swartz M. N. Genetic and biochemical characterization of norfloxacin resistance in Escherichia coli. Antimicrob Agents Chemother. 1986 Apr;29(4):639–644. doi: 10.1128/aac.29.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y., Sato K., Fujii T., Hirai K., Inoue M., Iyobe S., Mitsuhashi S. Some properties of subunits of DNA gyrase from Pseudomonas aeruginosa PAO1 and its nalidixic acid-resistant mutant. J Bacteriol. 1987 May;169(5):2322–2325. doi: 10.1128/jb.169.5.2322-2325.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M. I. Los Alamos sequence analysis package for nucleic acids and proteins. Nucleic Acids Res. 1982 Jan 11;10(1):183–196. doi: 10.1093/nar/10.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga H., Itoh A., Murayama S., Suzue S., Irikura T. Structure-activity relationships of antibacterial 6,7- and 7,8-disubstituted 1-alkyl-1,4-dihydro-4-oxoquinoline-3-carboxylic acids. J Med Chem. 1980 Dec;23(12):1358–1363. doi: 10.1021/jm00186a014. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- LESHER G. Y., FROELICH E. J., GRUETT M. D., BAILEY J. H., BRUNDAGE R. P. 1,8-NAPHTHYRIDINE DERIVATIVES. A NEW CLASS OF CHEMOTHERAPEUTIC AGENTS. J Med Pharm Chem. 1962 Sep;91:1063–1065. doi: 10.1021/jm01240a021. [DOI] [PubMed] [Google Scholar]
- Maiden M. C., Davis E. O., Baldwin S. A., Moore D. C., Henderson P. J. Mammalian and bacterial sugar transport proteins are homologous. Nature. 1987 Feb 12;325(6105):641–643. doi: 10.1038/325641a0. [DOI] [PubMed] [Google Scholar]
- Matsumoto J., Miyamoto T., Minamida A., Nishimura Y., Egawa H., Nishimura H. Pyridonecarboxylic acids as antibacterial agents. 2. Synthesis and structure-activity relationships of 1,6,7-trisubstituted 1,4-dihydro-4-oxo-1,8-naphthyridine-3-carboxylic acids, including enoxacin, a new antibacterial agent. J Med Chem. 1984 Mar;27(3):292–301. doi: 10.1021/jm00369a011. [DOI] [PubMed] [Google Scholar]
- McMurry L., Petrucci R. E., Jr, Levy S. B. Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3974–3977. doi: 10.1073/pnas.77.7.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Miyamoto T., Matsumoto J., Chiba K., Egawa H., Shibamori K., Minamida A., Nishimura Y., Okada H., Kataoka M., Fujita M. Synthesis and structure-activity relationships of 5-substituted 6,8-difluoroquinolones, including sparfloxacin, a new quinolone antibacterial agent with improved potency. J Med Chem. 1990 Jun;33(6):1645–1656. doi: 10.1021/jm00168a018. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Nakamura M., Kojima T., Yoshida H. gyrA and gyrB mutations in quinolone-resistant strains of Escherichia coli. Antimicrob Agents Chemother. 1989 Feb;33(2):254–255. doi: 10.1128/aac.33.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. T., Postle K., Bertrand K. P. Sequence homology between the tetracycline-resistance determinants of Tn10 and pBR322. Gene. 1983 Nov;25(1):83–92. doi: 10.1016/0378-1119(83)90170-1. [DOI] [PubMed] [Google Scholar]
- Rella M., Haas D. Resistance of Pseudomonas aeruginosa PAO to nalidixic acid and low levels of beta-lactam antibiotics: mapping of chromosomal genes. Antimicrob Agents Chemother. 1982 Aug;22(2):242–249. doi: 10.1128/aac.22.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard N. J., Scarpa A. L. Genetic and physiological characterization of ciprofloxacin resistance in Pseudomonas aeruginosa PAO. Antimicrob Agents Chemother. 1988 Apr;32(4):535–539. doi: 10.1128/aac.32.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Inoue Y., Fujii T., Aoyama H., Inoue M., Mitsuhashi S. Purification and properties of DNA gyrase from a fluoroquinolone-resistant strain of Escherichia coli. Antimicrob Agents Chemother. 1986 Nov;30(5):777–780. doi: 10.1128/aac.30.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalit I., Berger S. A., Gorea A., Frimerman H. Widespread quinolone resistance among methicillin-resistant Staphylococcus aureus isolates in a general hospital. Antimicrob Agents Chemother. 1989 Apr;33(4):593–594. doi: 10.1128/aac.33.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata K., Itoh-Yamashita N., Konno M. Cloning and expression of the norA gene for fluoroquinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1989 Sep;33(9):1535–1539. doi: 10.1128/aac.33.9.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie N. M., Clements J. B., Boll W., Mantei N., Lonsdale D., Weissmann C. Hybrid plasmids containing an active thymidine kinase gene of Herpes simplex virus 1. Nucleic Acids Res. 1979 Oct 25;7(4):859–877. doi: 10.1093/nar/7.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi J., Yoshida H., Yamayoshi M., Nakamura S. Nalidixic acid-resistant mutations of the gyrB gene of Escherichia coli. Mol Gen Genet. 1986 Sep;204(3):367–373. doi: 10.1007/BF00331012. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Bogaki M., Nakamura M., Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob Agents Chemother. 1990 Jun;34(6):1271–1272. doi: 10.1128/aac.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Kojima T., Yamagishi J., Nakamura S. Quinolone-resistant mutations of the gyrA gene of Escherichia coli. Mol Gen Genet. 1988 Jan;211(1):1–7. doi: 10.1007/BF00338386. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Nakamura M., Bogaki M., Nakamura S. Proportion of DNA gyrase mutants among quinolone-resistant strains of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 Jun;34(6):1273–1275. doi: 10.1128/aac.34.6.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]



