Abstract
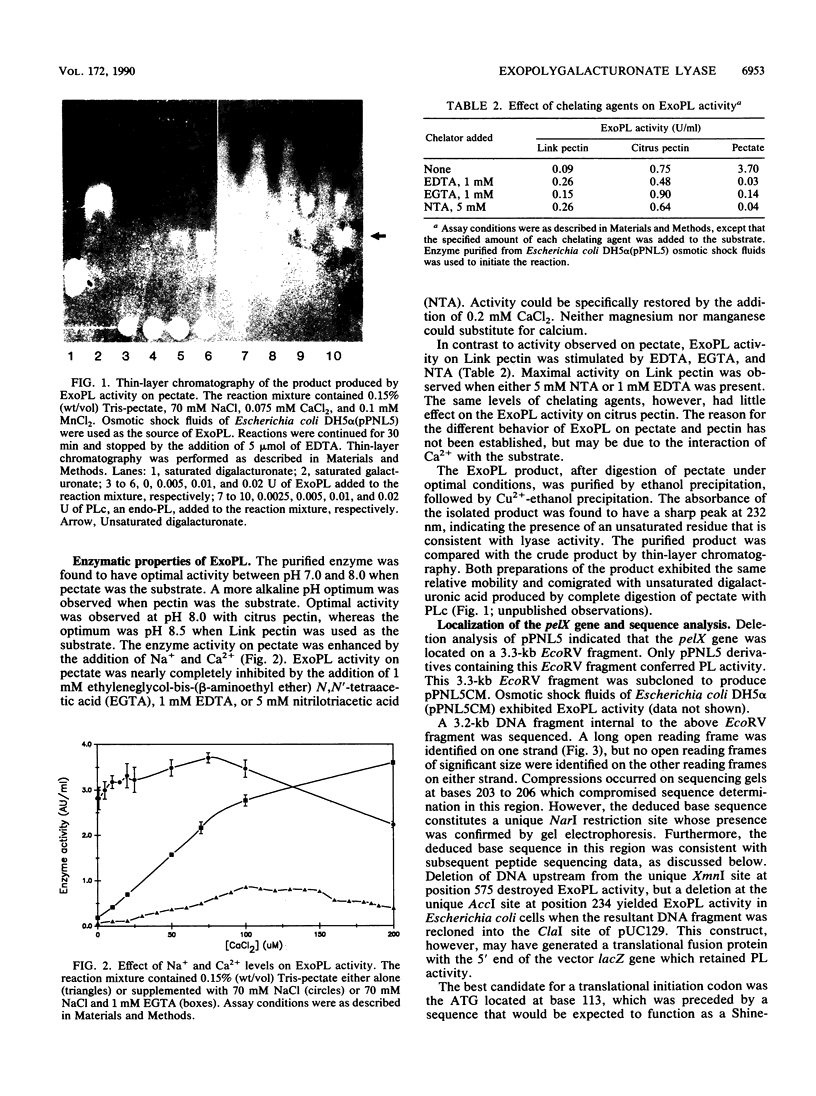
The ability of Erwinia chrysanthemi to cause soft-rot diseases involving tissue maceration in many plants has been linked to the production of endo-pectate lyase E. chrysanthemi EC16 mutant UM1005, however, contains deletions in the pel genes that encode the known endopectate lyases, yet still macerates plant tissues. In an attempt to identify the remaining macerating factor(s), a gene library of UM1005 was constructed in Escherichia coli and screened for pectolytic activity. A clone (pPNL5) was identified in this library that contained the structural gene for an exopolygalacturonate lyase (ExoPL). The gene for ExoPL was localized on a 3.3-kb EcoRV fragment which contained an open reading frame for a 79,500-Da polypeptide. ExoPL was purified to apparent homogeneity from Escherichia coli DH5 alpha (pPNL5) and found to have an apparent molecular weight of 76,000 with an isoelectric point of 8.6. Purified ExoPL had optimal activity between pH 7.5 and 8.0 and could utilize pectate, citrus pectin, and highly methyl-esterified Link pectin as substrates. A PL- ExoPL- mutant of EC16 was constructed that exhibited reduced growth on pectate, but retained pathogenicity on chrysanthemum equivalent to that of UM1005. The results indicate that ExoPL does not contribute to the residual macerating activity of UM1005.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barras F., Thurn K. K., Chatterjee A. K. Resolution of four pectate lyase structural genes of Erwinia chrysanthemi (EC16) and characterization of the enzymes produced in Escherichia coli. Mol Gen Genet. 1987 Sep;209(2):319–325. doi: 10.1007/BF00329660. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. K., Thurn K. K., Feese D. A. Tn5-Induced Mutations in the Enterobacterial Phytopathogen Erwinia chrysanthemi. Appl Environ Microbiol. 1983 Feb;45(2):644–650. doi: 10.1128/aem.45.2.644-650.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. K., Thurn K. K., Tyrell D. J. Isolation and characterization of Tn5 insertion mutants of Erwinia chrysanthemi that are deficient in polygalacturonate catabolic enzymes oligogalacturonate lyase and 3-deoxy-D-glycero-2,5-hexodiulosonate dehydrogenase. J Bacteriol. 1985 May;162(2):708–714. doi: 10.1128/jb.162.2.708-714.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collmer A., Bateman D. F. Impaired induction and self-catabolite repression of extracellular pectate lyase in Erwinia chrysanthemi mutants deficient in oligogalacturonide lyase. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3920–3924. doi: 10.1073/pnas.78.6.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collmer A., Whalen C. H., Beer S. V., Bateman D. F. An exo-poly-alpha-D-galacturonosidase implicated in the regulation of extracellular pectate lyase production in Erwinia chrysanthemi. J Bacteriol. 1982 Feb;149(2):626–634. doi: 10.1128/jb.149.2.626-634.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouy M., Gautier C. Codon usage in bacteria: correlation with gene expressivity. Nucleic Acids Res. 1982 Nov 25;10(22):7055–7074. doi: 10.1093/nar/10.22.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S. Y., Collmer A. Molecular cloning, nucleotide sequence, and marker exchange mutagenesis of the exo-poly-alpha-D-galacturonosidase-encoding pehX gene of Erwinia chrysanthemi EC16. J Bacteriol. 1990 Sep;172(9):4988–4995. doi: 10.1128/jb.172.9.4988-4995.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton J. C., Sidebotham J. M., Gill D. R., Salmond G. P. Extracellular and periplasmic isoenzymes of pectate lyase from Erwinia carotovora subspecies carotovora belong to different gene families. Mol Microbiol. 1989 Dec;3(12):1785–1795. doi: 10.1111/j.1365-2958.1989.tb00164.x. [DOI] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Keen N. T., Tamaki S., Kobayashi D., Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988 Oct 15;70(1):191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Min K. T., Kim M. H., Lee D. S. Search for the optimal sequence of the ribosome binding site by random oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1988 Jun 10;16(11):5075–5088. doi: 10.1093/nar/16.11.5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran F., Nasuno S., Starr M. P. Oligogalacturonide trans-eliminase of Erwinia carotovora. Arch Biochem Biophys. 1968 Jun;125(3):734–741. doi: 10.1016/0003-9861(68)90508-0. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Munson R. Separation of the inner (cytoplasmic) and outer membranes of Gram-negative bacteria. Methods Enzymol. 1974;31:642–653. doi: 10.1016/0076-6879(74)31070-1. [DOI] [PubMed] [Google Scholar]
- Payne J. H., Schoedel C., Keen N. T., Collmer A. Multiplication and Virulence in Plant Tissues of Escherichia coli Clones Producing Pectate Lyase Isozymes PLb and PLe at High Levels and of an Erwinia chrysanthemi Mutant Deficient in PLe. Appl Environ Microbiol. 1987 Oct;53(10):2315–2320. doi: 10.1128/aem.53.10.2315-2320.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley N. B., Reeves P. R. Chloramphenicol resistance cloning vector based on pUC9. Plasmid. 1987 Jan;17(1):54–57. doi: 10.1016/0147-619x(87)90008-4. [DOI] [PubMed] [Google Scholar]
- Reverchon S., Huang Y., Bourson C., Robert-Baudouy J. Nucleotide sequences of the Erwinia chrysanthemi ogl and pelE genes negatively regulated by the kdgR gene product. Gene. 1989 Dec 21;85(1):125–134. doi: 10.1016/0378-1119(89)90472-1. [DOI] [PubMed] [Google Scholar]
- Ried J. L., Collmer A. An nptI-sacB-sacR cartridge for constructing directed, unmarked mutations in gram-negative bacteria by marker exchange-eviction mutagenesis. Gene. 1987;57(2-3):239–246. doi: 10.1016/0378-1119(87)90127-2. [DOI] [PubMed] [Google Scholar]
- Ried J. L., Collmer A. Comparison of pectic enzymes produced by Erwinia chrysanthemi, Erwinia carotovora subsp. carotovora, and Erwinia carotovora subsp. atroseptica. Appl Environ Microbiol. 1986 Aug;52(2):305–310. doi: 10.1128/aem.52.2.305-310.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder D. L., Collmer A. Marker-exchange mutagenesis of a pectate lyase isozyme gene in Erwinia chrysanthemi. J Bacteriol. 1985 Oct;164(1):51–56. doi: 10.1128/jb.164.1.51-56.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STARR M. P., MORAN F. Eliminative split of pectic substances by phytopathogenic soft-rot bacteria. Science. 1962 Mar 16;135(3507):920–921. doi: 10.1126/science.135.3507.920. [DOI] [PubMed] [Google Scholar]
- Starr M. P., Chatterjee A. K., Starr P. B., Buchanan G. E. Enzymatic degradation of polygalacturonic acid by Yersinia and Klebsiella species in relation to clinical laboratory procedures. J Clin Microbiol. 1977 Oct;6(4):379–386. doi: 10.1128/jcm.6.4.379-386.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trollinger D., Berry S., Belser W., Keen N. T. Cloning and characterization of a pectate lyase gene from Erwinia carotovora EC153. Mol Plant Microbe Interact. 1989 Jan-Feb;2(1):17–25. doi: 10.1094/mpmi-2-017. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zink R. T., Engwall J. K., McEvoy J. L., Chatterjee A. K. recA is required in the induction of pectin lyase and carotovoricin in Erwinia carotovora subsp. carotovora. J Bacteriol. 1985 Oct;164(1):390–396. doi: 10.1128/jb.164.1.390-396.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]