Abstract
Antisense oligodeoxynucleotides offer potential as therapeutic agents to inhibit gene expression. Recent evidence indicates that oligodeoxynucleotides designed to target specific nucleic acid sequences can interact nonspecifically with proteins. This report describes the interactive capabilities of phosphorothioate oligodeoxynucleotides of defined sequence and length with two essential protein tyrosine receptors, flk-1 and epidermal growth factor receptor (EGFR), and their effects on receptor signaling in a transfected and tumor cell line, respectively. Phosphorothioate oligodeoxynucleotides bound to the cell surface, as demonstrated by fluorescence-activated cell-sorter analyses (FACS), and perturbed receptor activation in the presence and absence of cognate ligands, EGF (EGFR) and vascular endothelial growth factor (flk-1), in phosphorylation assays. Certain phosphorothioate oligodeoxynucleotides interacted relatively selectively with flk-1 and partially blocked the binding of specific anti-receptor monoclonal antibodies to target sites. They stimulated EGFR phosphorylation in the absence of EGF but antagonized ligand-mediated activation of EGFR and flk-1. In vivo studies showed that a nonspecific phosphorothioate oligodeoxynucleotide suppressed the growth of glioblastoma in a mouse model of tumorigenesis. These results emphasize the capacity of phosphorothioate oligodeoxynucleotides to interact with cells in a sequence-selective nonantisense manner, while associating with cellular membrane proteins in ways that can inhibit cellular metabolic activities.
Antisense technology has been exploited as a potential therapeutic approach for the treatment of a variety of disorders including AIDS and cancer (1). The antisense strategy is based on the premise that sequence-specific oligodeoxynucleotides can serve as inhibitors of gene expression by a direct interaction with their appropriate mRNA or DNA targets. To improve the effectiveness of antisense oligodeoxynucleotides as therapeutic reagents, much effort has been placed on the development of structurally modified oligomers. In particular, phosphorothioate oligodeoxynucleotides have been widely used antisense reagents (2). In this class of oligodeoxynucleotide, a sulfur atom is substituted for one of the nonbridging oxygen atoms bound to phosphorus. Phosphorothioates offer several advantages over phosphodiester oligodeoxynucleotides, including greater serum stability and nuclease resistance (3). In addition, phosphorothioates hybridize well to target mRNA and elicit RNase H activity, which cleaves the mRNA of the mRNA–DNA duplex. However, the polyanionic nature of phosphorothioate oligodeoxynucleotides has led, in some cases, to uncertainty regarding the extent to which observed biological effects stem from a strictly antisense mechanism. Evidence implicating phosphorothioate oligodeoxynucleotides in nonsequence-specific protein interactions, i.e., aptameric binding, has raised questions regarding their specificity for target mRNA (4).
Oligodeoxynucleotides interact with proteins in a complex manner that is dependent on charge, length sequence, and concentration (1, 5, 6). These interactions may result in protein–oligomer complexes that may markedly affect normal cellular physiology. For example, phosphorothioate oligodeoxynucleotides have been shown to form complexes with the heparin binding growth factors basic fibroblast growth factor, platelet-derived growth factor, and vascular endothelial growth factor (VEGF) [but not epidermal growth factor (EGF), which has a low affinity for heparin] (7). In the case of basic fibroblast growth factor, binding of the phosphorothioate oligomer is augmented when four contiguous guanosine residues are present. In studies with the tyrosine kinase receptor bcr-abl, Bergan et al. (8), have shown that the direct interaction of an oligomer with the protein leads to a reduction in the phosphorylation levels of both receptor and of downstream signaling proteins.
This report addresses questions regarding the nonsequence-specific interactions of phosphorothioate oligodeoxynucleotides and how their cell-surface binding may perturb protein activity. Several in vitro assays were performed, which showed that phosphorothioate oligodeoxynucleotides of defined sequence and length can affect the activity of two cell-surface expressed protein tyrosine kinase receptors, flk-1 and EGF receptor (EGFR). These receptors play an important functional role in normal and pathological cellular events (9, 10). The results show that oligodeoxynucleotides have the capacity to bind differentially to these receptors and elicit alterations in cellular tyrosine phosphorylation patterns in the presence and absence of their cognate ligands. The in vivo administration of one nonspecific phosphorothioate oligodeoxynucleotide was found to significantly inhibit growth of glioblastoma xenografts in nude mice. These results highlight the biological potency of nonsequence-specific phosphorothioate oligodeoxynucleotides and demonstrate their potential therapeutic efficacy.
MATERIALS AND METHODS
Phosphorothioate Oligodeoxynucleotides.
All oligonucleotides were synthesized by standard phosphoramidite chemistry and purified as described by Tonkinson and Stein (11). SdC28 and SdC18 have been previously described (5, 6). Other oligodeoxynucleotides used in these studies had the following sequences: #2 (G9CCGGGCCAT), #2T (G9TTGGGTTAT), and G6T12. Oligodeoxynucleotides were diluted in sterile PBS to a concentration of 1 mM, aliquoted, and stored frozen.
Cell Lines, Reagents, and Growth Assays.
The NIH 3T3 and KB cell lines were obtained from the American Type Culture Collection. The GBM-18 cell line (a gift from P. Fisher, Columbia University) was derived from a patient with Stage IV glioblastoma multiforme. These cells are highly tumorigenic in mouse xenografts, as described (12). C441, an NIH 3T3 cell line transfected with a chimeric mouse flk-1/human cfms receptor (13) was maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% calf serum (CS). The tumor lines KB and GBM-18 were grown in RPMI medium 1640 supplemented with 1 mM glutamine, antibiotics, and 10% fetal calf serum (FCS). Murine anti-flk-1 monoclonal antibodies (mAbs) 73, 115, and a rat-derived mAb, DC101, were raised against the extracellular portion of flk-1 using a soluble chimeric receptor form (14). These antibodies bind to the flk-1/fms transfected cell line, C441, and specifically to the flk-1 receptor as shown by Western blotting and/or immunoprecipitation. mAb DC101 has been shown to neutralize ligand-induced receptor phosphorylation (14) and suppress the growth of glioblastoma xenografts in nude mice (12).
Phosphorylation Assays and Western Blotting.
The phosphorylation assays and Western blot analyses with C441 and tumor cell lines were performed as described (14). Briefly, subconfluent C441 and KB cells were starved for 24 hr in DMEM containing 0.5% CS or serum-free medium [RPMI medium 1640 supplemented with 0.5% bovine serum albumin (BSA)], respectively. For neutralization assays, an equal number of cells were prebound with 1, 2, or 4 μM of the indicated oligodeoxynucleotides in serum-free medium for 10 min at room temperature. C441 cells were then stimulated with 20 ng/ml VEGF (Peprotech, Rocky Hill, NJ) for 10 min at room temperature, while KB cells were incubated with 10 ng/ml EGF (Sigma) for an additional 8 min. Where indicated, C441 cells were subjected to a cold PBS wash prior to the addition of VEGF. Following ligand stimulation, cells were washed with cold PBS containing 1 mM sodium orthovanadate, lysed in a buffer containing 20 mM Tris⋅HCl (pH 7.4), 1% N-octylglucoside, 137 mM NaCl, 10% (vol/vol) glycerol, 10 mM EDTA, 0.1 mM sodium orthovanadate, 10 mM NaF, 100 mM sodium pyrophosphate, 100 μg/ml Pefabloc (Boehringer Mannheim), 100 μg/ml aprotinin, and 100 μg/ml leupeptin. Following centrifugation at 14,000 × g for 10 min, receptors were immunoprecipitated from cleared lysates with protein A-Sepharose beads coupled to anti-receptor antibodies. EGFR was retrieved from KB lysates with a mAb (C225) raised against the N-terminal domain of the human EGF receptor (15), whereas a peptide-generated polyclonal antibody (IM133) to the C-terminal region of the c-fms receptor was used to immunoprecipitate flk-1/fms from C441 lysates. The beads were washed, mixed with SDS loading buffer, and subjected to Western blot analysis. The phosphoprotein patterns of the stimulated receptors were detected using an anti-phosphotyrosine mAb (Upstate Biotechnology) and developed by enhanced chemiluminescence (Amersham).
Immunofluorescent Detection of Blockade of mAb Binding.
For binding studies, C441 cells were removed with 2 mM EDTA in PBS, washed with cold Hanks’ balanced salt solution supplemented with 1% BSA, and then resuspended in 100 μl of the same buffer at a concentration of 105 cells per sample. Where indicated, cells were incubated with 2 μM oligodeoxynucleotides or 10 μg/ml heparin. After a 30-min incubation on ice, cells were washed and reincubated for 30 min with 10 μg of the appropriate mAb. After washing, a 1:40 dilution of goat anti-mouse IgG conjugated to fluorescein isothiocyanate (Tago) was added for a final 30-min incubation on ice. Cells were then analyzed on a Coulter Epics Elite Cytometer. Nonspecific binding was determined from samples incubated with oligodeoxynucleotide alone and an irrelevant melanoma-specific anti-gp75 mAb (TA99). Data are expressed as the measurement of the mean fluorescent intensity of mAb binding to cells pretreated with competitors relative to the control measurement of antibody binding to cells preincubated alone [mean fluorescent intensity (MFI) = 1] with the measurement of the irrelevant mAb subtracted as background fluorescence.
Animal Tumor Assays.
Animal studies were conducted with groups containing 5–10 athymic nude mice (nu/nu; Charles River Breeding Laboratories). Treatments were initiated 7 days after implantation of 1–2 × 106 GBM-18 cells per mouse. Animals were injected either intraperitoneally with mAb DC101 (200 μg antibody per mouse) or subcutaneously with oligodeoxynucleotides SdC28 and #2 (200 μl of a 1 μM solution in PBS per mouse) twice weekly for 3 weeks. PBS-treated mice served as controls. Tumor size was measured twice weekly with a caliper and tumor volume was calculated by the formula V = π/6 × larger diameter × (smaller diameter)2 (16).
RESULTS
Oligodeoxynucleotides Block VEGF-Induced Receptor Activation.
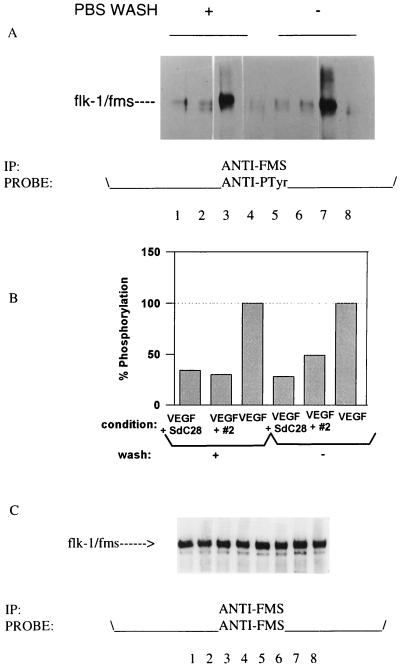
In these studies we examined nonspecific oligodeoxynucleotides for their ability to perturb ligand-induced activation of two cell-surface protein tyrosine kinase receptors, flk-1 and EGFR. To address the specificity of these interactions, phosphorothioate oligodeoxynucleotides were intially assayed for their effects on the VEGF-mediated activation of its cognate receptor expressed on the flk-1/fms transfected cell line, C441. In these assays, C441 cells were prebound with 2 μM oligodeoxynucleotide and stimulated with VEGF either before or after a PBS wash. The results (Fig. 1A) indicate that phosphorothioate oligodeoxynucleotides have the capacity to abrogate the VEGF-induced receptor phosphorylation of unwashed cells (Fig. 1A, lanes 5 and 6) to the level of unstimulated cells (Fig. 1A, lane 4). The ability of the oligodeoxynucleotides to do this is similar to that observed with a neutralizing anti-flk-1 mAb, DC101 (12). The extent of this inhibition can only be attributed, in part, to a previous demonstration that oligodeoxynucleotides bind VEGF in solution (7), since it is unlikely that an oligodeoxynucleotide concentration of 2 μM would elicit such a potent block of ligand binding to receptor. Nevertheless, the data also show that oligodeoxynucleotides remain bound to the C441 cell surface after washing and exert an apparent inhibition of receptor activation (Fig. 1A, lanes 1 and 2) relative to controls (lanes 3 and 7). A quantification of these effects on receptor–ligand interactions is shown in Fig. 1B. A reprobing of the blot with an anti-fms antibody (Fig. 1C) indicated that the observed effects on receptor activation were due to variations in protein phosphorylation rather than differences in the levels of receptor loaded per lane. Similar inhibitory effects were observed for two additional oligodeoxynucleotides, #2T and SdG6T12 (a tetraplex forming oligodeoxynucleotide), whereas none of the oligodeoxynucleotides alone exerted an effect on the activation of the flk-1/fms receptor when assayed in the absence of ligand (data not shown).
Figure 1.
(A) Effect of phosphorothioate oligodeoxynucleotides on VEGF-induced activation of the flk-1/fms receptor. Each lane represents the phosphorylated receptor immunoprecipitated from an equal number of C441 cells prebound with 2 μM oligodeoxynucleotide and stimulated with 20 ng/ml VEGF for 15 min at room temperature. Cells were either washed with cold PBS (lanes 1–4) or unwashed (lanes 5–8) prior to the addition of ligand. The level of flk-1/fms activation induced by VEGF is shown for cells preincubated with 2 μM of the oligodeoxynucleotides SdC28 (lanes 1 and 5), #2 (lanes 2 and 6), or without oligodeoxynucleotide (lanes 3 and 7) relative to unstimulated controls (lanes 4 and 8). (B) A densitometry scan of A. Data plotted showing flk-1/fms phosphorylation levels with the measurement for the unstimulated control subtracted. (C) A reprobing of the blot with an anti-fms polyclonal antibody showing the same level of receptor in each lane.
Oligodeoxynucleotides Elicit Agonist and Antagonist Effects on EGFR Activity.
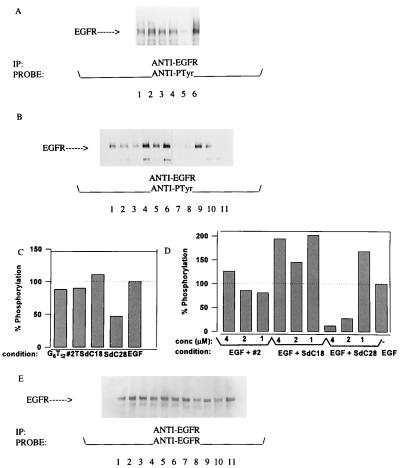
Similar studies were performed on the activation of EGFR expressed in the epidermoid tumor cell line (KB). Since oligodeoxynucleotides do not appear to directly bind to EGF (7), these experiments preclude the possibility that perturbations of receptor kinase activity reflect direct ligand–oligodeoxynucleotide interactions in solution. Our results indicate that oligodeoxynucleotides can either act specifically as agonists or antagonists of EGFR activation depending on the presence or absence of ligand (Fig. 2). In the absence of EGF, increased phosphoprotein patterns were observed in immunoprecipitates of EGFR when KB cells were incubated with either the SdC28, SdC18, #2T, or SdG6T12 oligodeoxynucleotides alone (Fig. 2A). A different effect on EGFR activation was observed when oligodeoxynucleotides were assayed in the presence of ligand (Fig. 2B). A study of the dose dependency (1 μM, 2 μM, and 4 μM) of this effect demonstrated that potent inhibition of ligand activation was achieved by SdC28, but not by either SdC18 or #2 oligodeoxynucleotides, at a final concentration of 2 μM. Quantifications of the results for Fig. 2 A and B are shown relative to that obtained for EGF alone in Fig. 2 C and D. The ability of SdC18 to enhance EGF-induced phosphorylation (Fig. 2D) was found to vary between experiments. Verification that the changes in receptor phosphorylation corresponded to equal amounts of EGFR per lane (Fig. 2E) was obtained by reprobing the blots with an anti-receptor polyclonal antibody (Upstate Biotechnology). It should be noted that these effects (i.e., binding to cell-surface proteins) occur despite the fact that all assay media are supplemented with either 0.5% or 1.0% BSA. Furthermore, neither #2T nor SdG6T12 oligodeoxynucleotide-inhibited EGF induced receptor phosphorylation (data not shown). In cellular proliferation assays, a similar trend of inhibition was observed on starved KB cells as oligodeoxynucleotides (SdC28, #2, #2T, and G6T12) were found to induce differential changes in cell morphology after a 72-hr period of incubation (data not shown).
Figure 2.
(A) Effect of phosphorothioate oligodeoxynucleotides on EGFR activity in KB cells. Shown is oligodeoxynucleotide-induced phosphorylation of EGFR immunoprecipitated from lysates of KB cells following a 10-min incubation at room temperature with 2 μM of the oligodeoxynucleotides G6T12 (lane 1), #2T (lane 2), SdC18 (lane 3), SdC28 (lane 4), or without oligodeoxynucleotide in the absence (lane 5) and presence (lane 6) of EGF. (B) Effect of oligodeoxynucleotides on EGF-induced activation of EGFR. The level of receptor phosphorylation is shown for EGF (10 ng/ml)-stimulated cells preincubated with 4 μM, 2 μM, and 1 μM of the oligodeoxynucleotides #2 (lanes 1–3), SdC18 (lanes 4–6), SdC28 (lanes 7–9) versus EGF-stimulated cells (lane 10) and unstimulated cells (lane 11). (C and D) Densitometry scans of the EGFR phosphorylation shown in A and B, respectively, plotted with the unstimulated control measurements subtracted. (E) Blots from B reprobed with an anti-EGFR polyclonal antibody showing the same level of receptor per lane.
Oligodeoxynucleotides Block Cell-Surface Receptor Binding by Anti-flk-1 mAbs.
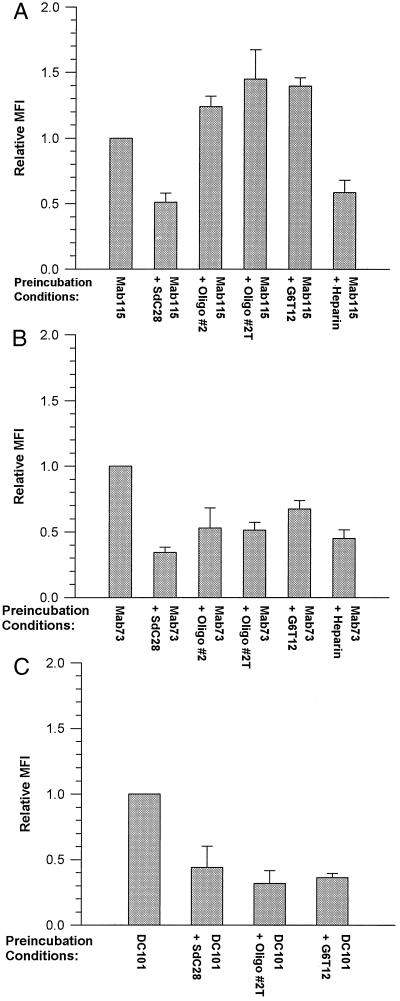
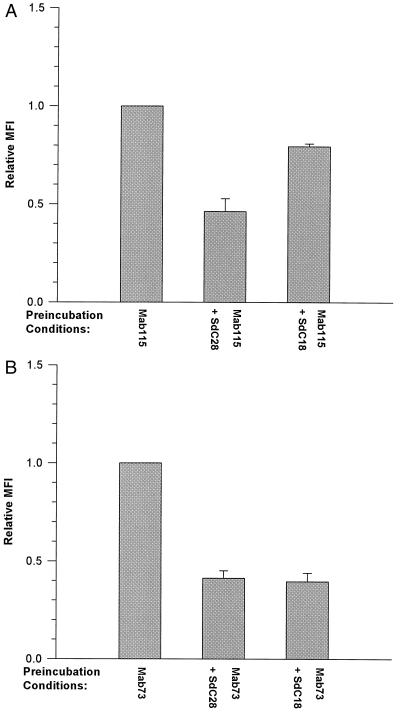
A direct demonstration of the interactions of oligodeoxynucleotides with cell-surface proteins was obtained from FACS analyses showing oligodeoxynucleotide-induced blockade of the binding of three anti-flk-1 mAbs, each possessing different affinities for the receptor (ref. 14 and unpublished data). The demonstration that the observed nonsequence-specific effects were exerted at the cell surface was insured by performing these binding assays under conditions (at 4°C) that minimized receptor internalization. These experiments indicate that oligodeoxynucleotides vary in their effects on mAb binding to C441 cells. The mean fluorescent intensity of mAb binding to oligodeoxynucleotide-treated cells relative to cells bound by mAb alone is shown in Fig. 3. SdC28 elicited a partial block of the binding of mAbs 115 (Fig. 3A), 73 (Fig. 3B), and DC101 (Fig. 3C) to C441 cells. A similar level of inhibition was also elicited by the #2, #2T, and SdG6T12 oligodeoxynucleotides on mAb DC101 binding, whereas the binding of mAb 73 was blocked to a lesser extent by these oligodeoxynucleotides. Conversely, #2T and SdG6T12 enhanced the cell-surface receptor binding of mAb 115. We have also included data on the inhibition of mAbs 115 and 73 binding by 10 μg/ml heparin as a comparison with the effects observed with phosphorothioate oligodeoxynucleotides. The greater inhibition (an ≈40% decrease) of mAb 115 binding observed with SdC28 relative to SdC18 (Fig. 4A) is evidence that blockade of binding is determined by oligodeoxynucleotide length. This differential effect was not obvious with mAb 73 (Fig. 4B), which was more sensitive to inhibition of binding by both oligodeoxynucleotides.
Figure 3.
FACS analysis of phosphorothioate oligodeoxynucleotide blockade of mAb binding to cell-surface targets. Shown is the binding of anti-flk-1 mAbs 115 (A), 73 (B), and DC101 (C) to C441 cells preincubated with either 2 μM of the oligodeoxynucleotides SdC28, #2, #2T, G6T12, or 10 μg/ml heparin or without oligodeoxynucleotide. All incubations were performed at 4°C to prevent flk-1 receptor internalization. The specific preincubation conditions used for each mAb-binding experiment are indicated in the appropriate figure. Each graph represents the MFI of mAb binding to cells pretreated with oligodeoxynucleotide or heparin relative to antibody binding alone on cells preincubated without competitors (normalized to MFI = 1). Measurements obtained with the irrelevant control are subtracted as background fluorescence. Data are averages of the relative MFI (± SEM) from at least three independent experiments. With the exception of mAb 115 binding to #2 treated cells in A, a statistical analysis (t test) of the data showed that mAb binding to cells pretreated with either oligodeoxynucleotide or heparin were significantly different (P < 0.05) from their respective controls.
Figure 4.
FACS analysis of mAb binding to C441 cells preincubated with oligodeoxynucleotides of identical sequence but different length. Shown are the binding of mAbs 115 (A) and 73 (B) to cells prebound with either SdC28 or SdC18. Results are graphed and analyzed as described in the legend to Fig. 5. mAb binding to oligodeoxynucleotide-treated cells was significantly different from control values of binding to untreated cells (P < 0.05).
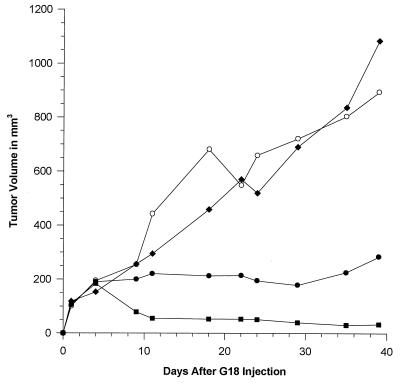
SdC28 Suppresses Tumor Growth.
To assess their potential therapeutic efficacy, SdC28 and #2 were examined for their ability to suppress the growth of human glioblastoma xenografts in nude mice (Fig. 5). These studies included an anti-flk-1 mAb (DC101), which has been shown to induce a significant inhibition of glioblastoma growth in this tumor model (12). Our results indicate that the SdC28 and mAb DC101, but not #2, induced a significant inhibition (P < 0.001) of tumor growth relative to that of the PBS control. There was no mortality nor clinical evidence of toxicity in any of the test groups. Although the effects of mAb DC101 can presumably be attributed to an inhibition of tumor angiogenesis (12, 14) it is difficult to ascertain the basis for the oligodeoxynucleotide suppression of tumor growth in vivo due to the pleiotropic effects displayed in vitro. Interestingly, 1–2 μM of SdC28 elicited growth-inhibitory effects in cell-proliferation assays on endothelial (HUVEC), KB, and glioblastoma (GBM-18) cells, whereas #2 but not the SdC18 oligodeoxynucleotide elicited a similar inhibitory effect when assayed at higher concentrations (data not shown). The potency of the SdC28-induced inhibition is consistent with the results obtained from KB phosphorylation assays as well as previous data (1, 5).
Figure 5.
Effect of phosphorothioate oligodeoxynucleotides SdC28 and #2 on established tumor growth in a mouse model of human glioblastoma. Treatments were initiated 7 days after implantation of GBM-18 cells. Results are plotted as the tumor volume over time for each treatment: (○), PBS; (⧫), oligodeoxynucleotide #2; (•), DC101; (▪), oligodeoxynucleotide SdC28. A statistical analysis of a regression of the data points for each animal group over time (regressed line not shown on graph) showed that the rate of tumor growth (slope of each regression) for SdC28 and mAb DC101 was significantly different from the PBS control (P < 0.001).
DISCUSSION
The results presented in this report show that phosphorothioate oligodeoxynucleotides interact nonsequence-specifically with the cell-surface expressed protein tyrosine kinase receptors, flk-1 and EGFR, and perturb their ligand-induced activation. The specificity of each oligodeoxynucleotide–receptor interaction is addressed by immunoprecipitations with the appropriate antibodies from the lysates of treated cells. These studies indicated that oligodeoxynucleotides designed without sequence-specific targets have the capacity to elicit a complex series of effects on cell-surface expressed receptors. The ability of oligodeoxynucleotides to elicit agonist and antagonist effects on receptor tyrosine kinase activation differed as a function of receptor type (flk-1 versus EGFR). In each case, however, the oligodeoxynucleotide SdC28 induced a potent inhibition of ligand-mediated receptor phosphorylation.
The variations in receptor phosphorylation and cell-surface binding efficiencies elicited by phosphorothioate oligodeoxynucleotides may be attributed to their sequence and length. The oligomers differentially inhibit binding of anti-flk-1 mAbs to their targets. Thus, an antibody that displays a greater binding efficiency for its target based on MFI (mAbs 115 > 73 and DC101) may more readily displace a competitor from binding sites on the cell surface. The finding that inhibition of mAb binding and receptor phosphorylation is still evident after washing cells prebound with oligodeoxynucleotides suggests that phosphorothioate oligodeoxynucleotides are bound with high affinity to the cell surface in quantity sufficient to perturb cellular activities. A comparison of the effects exhibited by SdC18 versus SdC28 suggests that oligodeoxynucleotide length plays a role in eliciting more potent effects on receptor activation. Our data obtained from the FACS analyses, the phosphorylation assays with KB cells, and from previous reports (1, 5–7) demonstrate that the longer oligodeoxynucleotide SdC28 may bind more avidly than SdC18 to proteins. Nevertheless, the similarity in sensitivity of mAb 73 to binding inhibition by SdC28 and SdC18 in FACS analyses also suggests that the effect of oligodeoxynucleotide length on mAb binding may vary considerably depending on the affinity of the mAb for its receptor.
The sensitivity of the flk-1/fms to inhibition by phosphorothioate oligodeoxynucleotides resembles the previously demonstrated block of this receptor’s activation by heparin (13) and demonstrates that phosphorothioate oligodeoxynucleotides have properties similar to several naturally occurring polyanions, including heparin (5–7). In addition, the agonist effects elicited by oligodeoxynucleotides on EGFR activity are reminiscent of previous studies demonstrating that heparin alone can trigger the phosphorylation of this receptor (17) as well as the activation of the fibroblast growth factor 4 receptor (18). It should be kept in mind that phosphorothioate oligodeoxynucleotides with specific sequences have been shown to elicit nonantisense effects; for example, constructs with four contiguous guanine residues (G quartet) may display a high affinity for proteins and CpG motifs can induce cellular immune responses (19). A more recent study shows that 24-hr treatments with 25 μM of a sequence-specific phosphorothioate oligodeoxynucleotide designed to block EGFR mRNA expression specifically inhibited receptor kinase activity via a nonantisense mechanism of action (20). However, it is not clear whether the observed changes in phosphorylation are the consequence of a direct binding of oligodeoxynucleotide to receptor. It remains possible that interactions of oligodeoxynucleotides with associated kinases or phosphatases could indirectly alter receptor phosphoprotein patterns.
Given the overlap of these oligodeoxynucleotides for anti-flk-1 targets on transfected cells and the inhibitory capacity of mAb DC101 and mAb 73 in vivo (ref. 12, unpublished results), we anticipated a suppression of GBM-18 tumor growth as observed in SdC28 treated mice. This reduction in tumor growth, however, is only elicited by SdC28 and not by an eqimolar concentration of the #2 oligodeoxynucleotide, even though both were inhibitory in vitro. Thus, it is difficult to predict which of the various effects elicited by SdC28 in vitro are responsible for its potent suppression of glioblastoma tumor growth in vivo. Given their pleiotropic behavior in vitro, it is reasonable to assume that phosphorothioate oligodeoxynucleotides use multiple mechanisms of action to produce their antiproliferative effects in vivo. Inhibition of receptor phosphorylation and proliferation in vitro may represent only one of several mechanisms contributing to the potent inhibition exhibited by SdC28 at low concentrations in vivo. Phosphorothioate oligodeoxynucleotides may also behave as anti-angiogenic and/or anti-tumor reagents if their protein interactions result in blocking ligand-mediated activation of receptors on tumor and endothelial cells. Evidence to support this possibility stems from studies showing that ligand–oligodeoxynucleotide binding occurs for VEGF and fibroblast growth factor (7) and that phosphorothioate oligodeoxynucleotides suppress human melanoma growth in vivo via a nonantisense mechanism probably involving a basic fibroblast growth factor–oligodeoxynucleotide interaction (21).
Our data also suggests that nonsequence-specific oligodeoxynucleotides can perturb the activity of EGFR at the cell surface within a short time period (15 min). The ability of SdC28 to inhibit EGF-mediated phosphorylation of EGFR may explain at least, in part, the recent results of Wang et al. (22). These authors noted that SdC28 inhibited human aortic smooth muscle cell proliferation induced by EGF (63% inhibition at 10 μM). In addition, they correlated this inhibition with a dramatic SdC28-induced decrease in intimal hyperplasia in the rat carotid balloon injury model. These data raise the possibility that SdC28 may be useful as a clinical antirestenotic agent. Regardless of mechanism, it is possible that perturbations of cell-surface expressed receptors by oligodeoxynucleotides may have profound consequences on downstream signaling events that regulate fundamental physiological processes such as cell proliferation or migration.
The fact that our above observations result from nonantisense interactions does not detract from the therapeutic potential of phosphorothioate oligodeoxynucleotides as antisense anticancer reagents. The fact that they can also function in a sequence-specific manner (23, 24), and can down-regulate targeted expression does not preclude their synchronous capacity for nonsequence specificity. Clearly, stringent criteria must be established (25) to determine whether an observed biological effect results from a true antisense mechanism. Many biological endpoints attributed to antisense activity may, in reality, be the result of the interaction between nucleic acid and protein, as shown in this work.
Acknowledgments
We thank Allison Glassman and Ratchanee Songsakphisarn for technical assistance, Marie Prewett for graph preparations, and R. F. Rockwell for statistical advice. This work was partially funded by National Cancer Institute Grant R29 60639.
ABBREVIATIONS
- VEGF
vascular endothelial growth factor
- EGF
epidermal growth factor
- EGFR
EGF receptor
- FACS
fluorescence-activated cell sorter
- MFI
mean fluorescent intensity
References
- 1.Stein C A, Cheng Y-C. Science. 1993;261:1004–1012. doi: 10.1126/science.8351515. [DOI] [PubMed] [Google Scholar]
- 2.Stec W J, Zon G, Egan W, Stec B. J Am Chem Soc. 1984;106:6077–6079. [Google Scholar]
- 3.Stein C A, Subasinghe C, Shinozuka K, Cohen J. Nucleic Acids Res. 1988;16:3209–3221. doi: 10.1093/nar/16.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stein C A. Nat Med. 1995;1:119–121. doi: 10.1038/nm1195-1119. [DOI] [PubMed] [Google Scholar]
- 5.Khaled Z, Rideout D, O’Driscoll K R, Petrylak D, Cacace A, Patel R, Chiang L, Rotenberg S, Stein C A. Clin Cancer Res. 1995;1:113–122. [PubMed] [Google Scholar]
- 6.Yakubov L, Khaled Z, Zhang L-M, Truneh A, Vlassov V, Stein C A. J Biol Chem. 1993;268:18818–18823. [PubMed] [Google Scholar]
- 7.Guvakova M A, Yakubov L, Vlodavsky I, Tonkinson J L, Stein C A. J Biol Chem. 1995;270:2620–2627. doi: 10.1074/jbc.270.6.2620. [DOI] [PubMed] [Google Scholar]
- 8.Bergan R C, Kyle E, Connell Y, Neckers L. Antisense Res Dev. 1995;5:33–38. doi: 10.1089/ard.1995.5.33. [DOI] [PubMed] [Google Scholar]
- 9.Aaronson S A. Science. 1991;254:1146–1153. doi: 10.1126/science.1659742. [DOI] [PubMed] [Google Scholar]
- 10.Shibuya M. Adv Cancer Res. 1995;67:281–316. doi: 10.1016/s0065-230x(08)60716-2. [DOI] [PubMed] [Google Scholar]
- 11.Tonkinson J L, Stein C A. Nucleic Acids Res. 1994;22:4268–4275. doi: 10.1093/nar/22.20.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rockwell P, Goldstein N I. Mol Cell Diff. 1995;3:315–335. [Google Scholar]
- 13.Tessler S, Rockwell P, Hicklin D, Cohen T, Levi B-Z, Witte L, Lemischka I R, Neufeld G. J Biol Chem. 1994;269:12456–12461. [PubMed] [Google Scholar]
- 14.Rockwell P, Neufeld G, Glassman A, Caron D, Goldstein N. Mol Cell Diff. 1995;3:91–109. [Google Scholar]
- 15.Goldstein N I, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Clin Cancer Res. 1995;1:1311–1318. [PubMed] [Google Scholar]
- 16.Baselga J, Norton L, Masui H, Pandiella A, Coplan K, Miller W H, Jr, Mendelsonn J. J Natl Cancer Inst. 1993;85:1327–1333. doi: 10.1093/jnci/85.16.1327. [DOI] [PubMed] [Google Scholar]
- 17.Borowski P, Medem S, Lauff R, Weber W. J Biochem. 1994;115:825–829. doi: 10.1093/oxfordjournals.jbchem.a124423. [DOI] [PubMed] [Google Scholar]
- 18.Gao G, Goldfarb M. EMBO J. 1995;14:2183–2190. doi: 10.1002/j.1460-2075.1995.tb07212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krieg A M, Yi A-K, Matson S, Waldschmidt T J, Bishop G A, Teasdale R, Koretzky G A, Kliman D M. Nature (London) 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 20.Coulson J M, Poyner D R, Chantry A, Irwin W J, Akhtar S. Mol Pharmacol. 1996;50:314–325. [PubMed] [Google Scholar]
- 21.Jansen B, Wadl H, Inoue S A, Trulzsch B, Selzer E, Duchene M, Eichler H-G, Wolff K, Pehamberger H. Antisense Res Dev. 1995;5:271–277. doi: 10.1089/ard.1995.5.271. [DOI] [PubMed] [Google Scholar]
- 22.Wang W, Chen H, Schwartz A, Cannon P, Stein C A, Rabbani L. J Clin Invest. 1996;98:443–450. doi: 10.1172/JCI118810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monia B, Johnston J, Geiger T, Muller M, Fabbro D. Nat Med. 1996;2:668–675. doi: 10.1038/nm0696-668. [DOI] [PubMed] [Google Scholar]
- 24.Dean N, McKay R. Proc Natl Acad Sci USA. 1994;91:11762–11766. doi: 10.1073/pnas.91.24.11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein C A, Krieg A. Antisense Res Dev. 1994;4:67–69. doi: 10.1089/ard.1994.4.67. [DOI] [PubMed] [Google Scholar]