Abstract
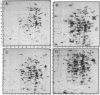
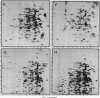
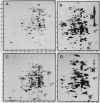
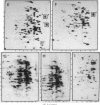
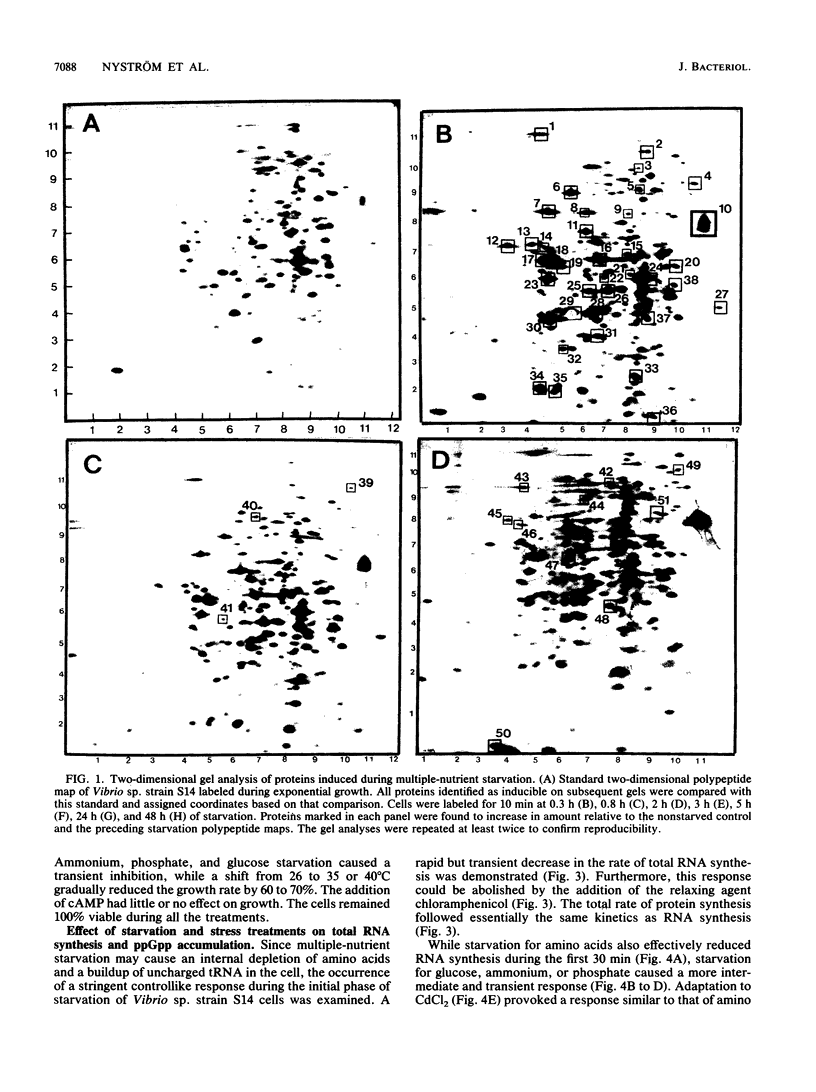
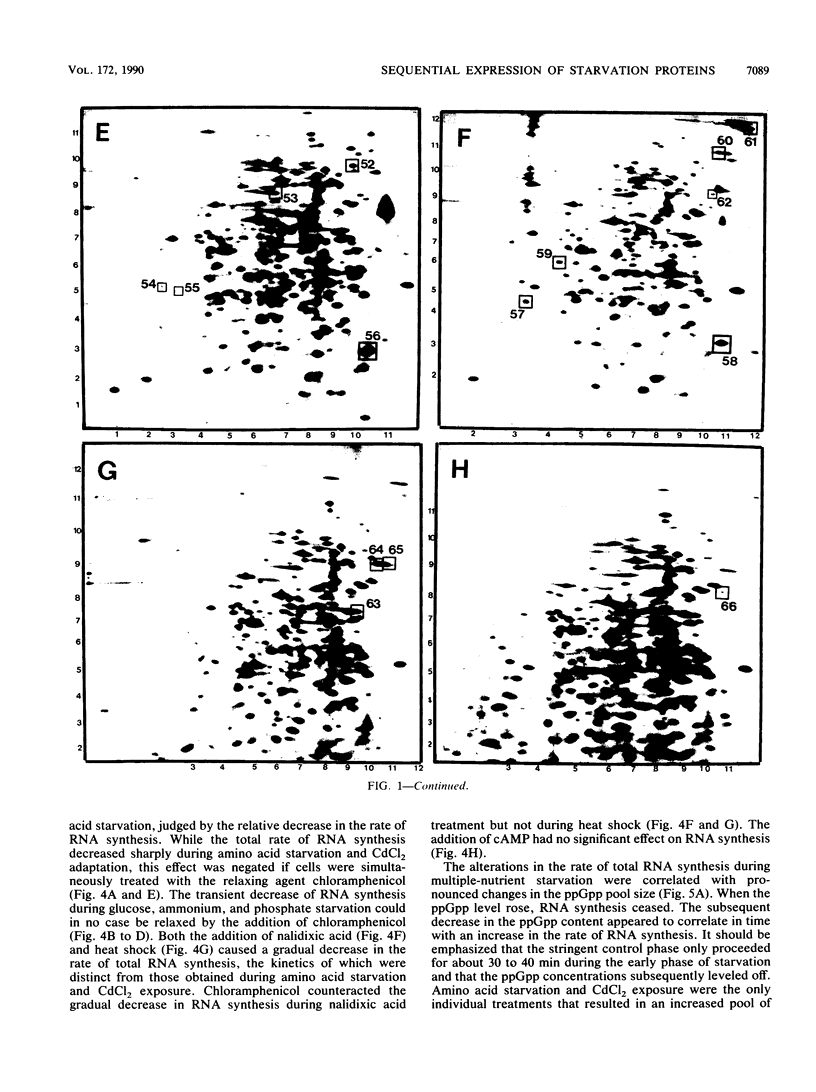
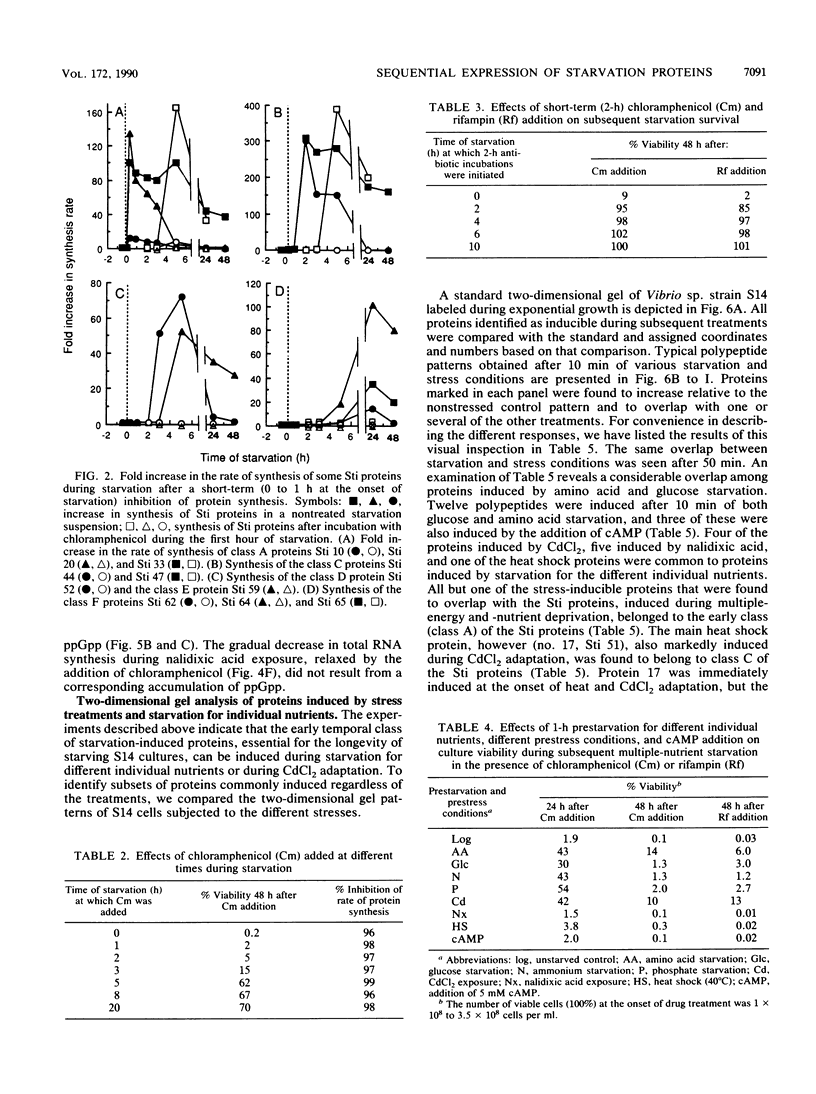
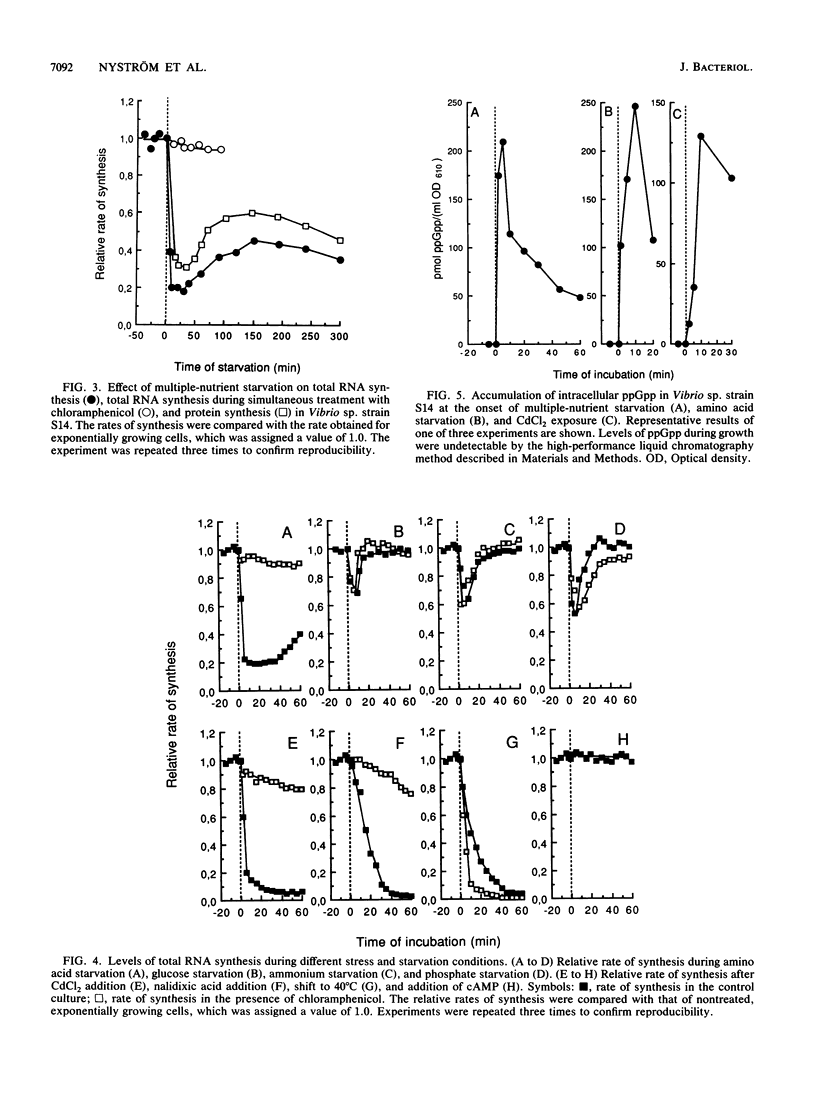
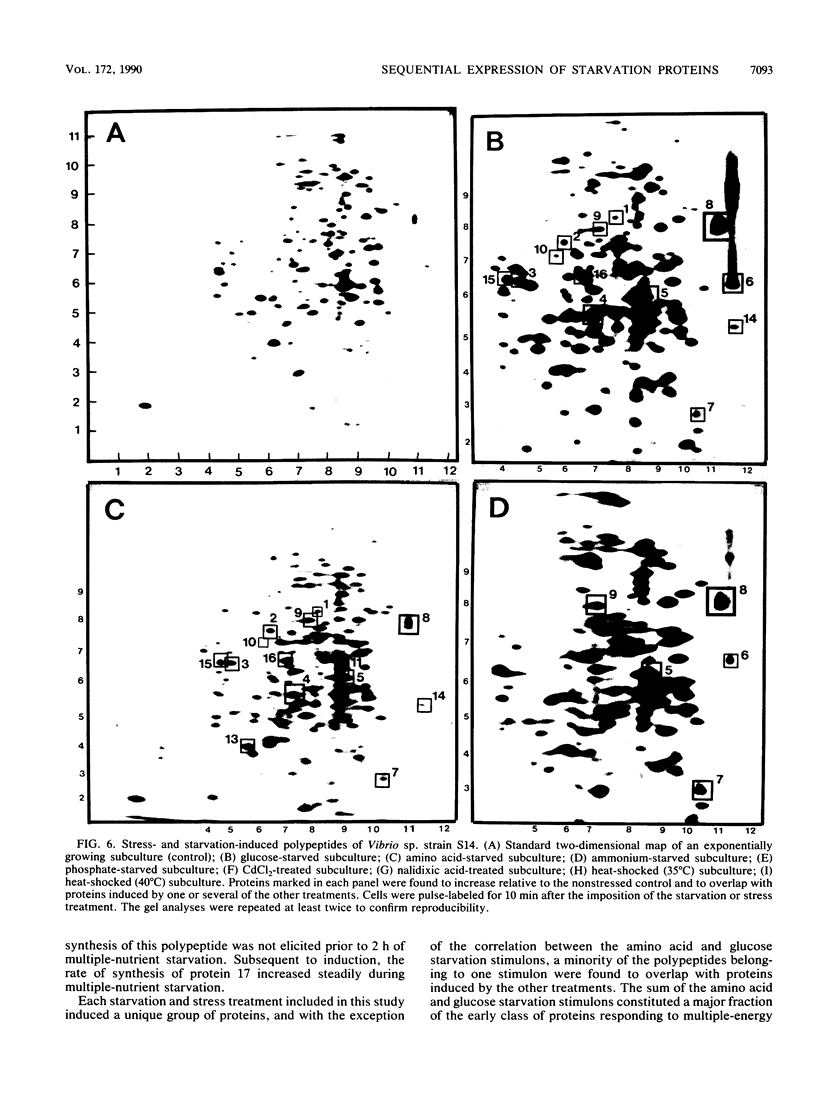
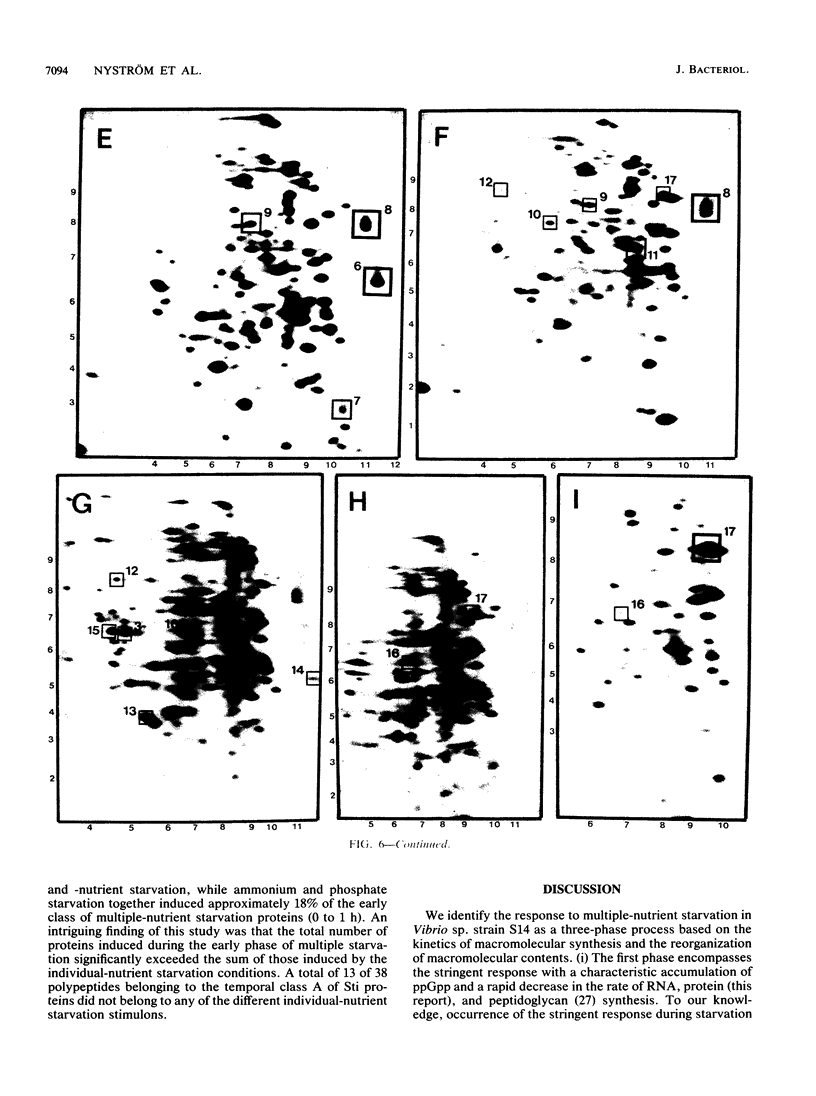
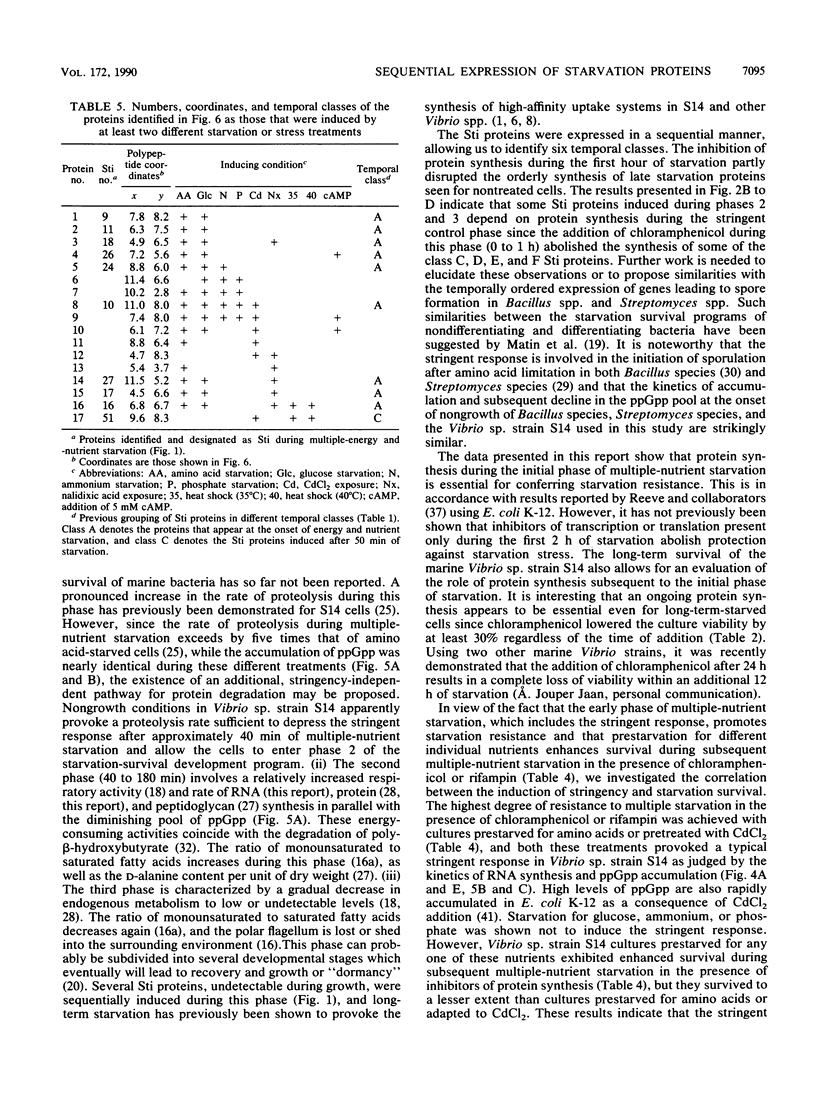
The response of marine Vibrio sp. strain S14 (CCUG 15956) to long-term (48-h) multiple-nutrient starvation (i.e., starvation for glucose, amino acids, ammonium, and phosphate simultaneously) can be described as a three-phase process. The first phase, defined as the stringent control phase, encompasses an accumulation of guanosine 5'-diphosphate 3'-diphosphate (ppGpp) and decreases in RNA and protein synthesis during the first 40 min. In the second phase, there is a temporary increase in the rates of RNA and protein synthesis between 1 and 3 h paralleling a decrease in the ppGpp pool. The third phase includes gradual decline in macromolecular synthesis after 3 h. Using two-dimensional gel electrophoresis of pulse-labeled proteins, a total of 66 proteins were identified as starvation inducible (Sti), temporally expressed throughout the three phases of starvation. The inhibition of protein synthesis during the first phase of starvation partly disrupted the subsequent temporally ordered synthesis of starvation proteins and prevented the expression of some late starvation proteins. It was also found that the early temporal class of starvation proteins, which included the majority of the Sti proteins, was the most essential for long-term survival. Vibrio sp. strain S14 cultures prestarved (1 h) for glucose, amino acids, ammonium, or phosphate as well as cultures exposed (1 h) to CdCl2 exhibited enhanced survival during the subsequent multiple-nutrient starvation in the presence of chloramphenicol or rifampin, while heat or the addition of cyclic AMP or nalidixic acid prior to starvation had no effect. It was demonstrated that amino acid starvation and CdCl2 exposure, which induced the stringent response, were the most effective in conferring enhanced survival. A few Sti proteins were common to all starvation conditions. In addition, the total number of proteins induced by multiple-nutrient starvation significantly exceeded the sum of those induced by starvation for each of the individual nutrients.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertson N. H., Nyström T., Kjelleberg S. Starvation-induced modulations in binding protein-dependent glucose transport by the marine Vibrio sp. S14. FEMS Microbiol Lett. 1990 Jul;58(2):205–209. doi: 10.1111/j.1574-6968.1990.tb13979.x. [DOI] [PubMed] [Google Scholar]
- Amy P. S., Morita R. Y. Protein Patterns of Growing and Starved Cells of a Marine Vibrio sp. Appl Environ Microbiol. 1983 Jun;45(6):1748–1752. doi: 10.1128/aem.45.6.1748-1752.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amy P. S., Pauling C., Morita R. Y. Starvation-survival processes of a marine Vibrio. Appl Environ Microbiol. 1983 Mar;45(3):1041–1048. doi: 10.1128/aem.45.3.1041-1048.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R. M., Singleton F. L., Hood M. A. Effects of nutrient deprivation on Vibrio cholerae. Appl Environ Microbiol. 1983 Oct;46(4):930–940. doi: 10.1128/aem.46.4.930-940.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belas R., Simon M., Silverman M. Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. J Bacteriol. 1986 Jul;167(1):210–218. doi: 10.1128/jb.167.1.210-218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. L., Robb F. T. Maintenance of Different Mannitol Uptake Systems during Starvation in Oxidative and Fermentative Marine Bacteria. Appl Environ Microbiol. 1985 Oct;50(4):743–748. doi: 10.1128/aem.50.4.743-748.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Spector M. P. Phosphate starvation regulon of Salmonella typhimurium. J Bacteriol. 1986 May;166(2):666–669. doi: 10.1128/jb.166.2.666-669.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groat R. G., Schultz J. E., Zychlinsky E., Bockman A., Matin A. Starvation proteins in Escherichia coli: kinetics of synthesis and role in starvation survival. J Bacteriol. 1986 Nov;168(2):486–493. doi: 10.1128/jb.168.2.486-493.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelleberg S., Hermansson M., Mårdén P., Jones G. W. The transient phase between growth and nongrowth of heterotrophic bacteria, with emphasis on the marine environment. Annu Rev Microbiol. 1987;41:25–49. doi: 10.1146/annurev.mi.41.100187.000325. [DOI] [PubMed] [Google Scholar]
- Kjelleberg S., Humphrey B. A., Marshall K. C. Effect of interfaces on small, starved marine bacteria. Appl Environ Microbiol. 1982 May;43(5):1166–1172. doi: 10.1128/aem.43.5.1166-1172.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little R., Bremer H. Quantitation of guanosine 5',3'-bisdiphosphate in extracts from bacterial cells by ion-pair reverse-phase high-performance liquid chromatography. Anal Biochem. 1982 Nov 1;126(2):381–388. doi: 10.1016/0003-2697(82)90531-0. [DOI] [PubMed] [Google Scholar]
- Mach H., Hecker M., Hill I., Schroeter A., Mach F. Physiologische Bedeutung der "Stringent Control" bei Escherichia coli unter extremen Hungerbedingungen. Z Naturforsch C. 1989 Sep-Oct;44(9-10):838–844. [PubMed] [Google Scholar]
- Malmcrona-Friberg K., Goodman A., Kjelleberg S. Chemotactic Responses of Marine Vibrio sp. Strain S14 (CCUG 15956) to Low-Molecular-Weight Substances under Starvation and Recovery Conditions. Appl Environ Microbiol. 1990 Dec;56(12):3699–3704. doi: 10.1128/aem.56.12.3699-3704.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A., Auger E. A., Blum P. H., Schultz J. E. Genetic basis of starvation survival in nondifferentiating bacteria. Annu Rev Microbiol. 1989;43:293–316. doi: 10.1146/annurev.mi.43.100189.001453. [DOI] [PubMed] [Google Scholar]
- Mosteller R. D. Evidence that glucose starvation-sensitive mutants are altered in the relB locus. J Bacteriol. 1978 Feb;133(2):1034–1037. doi: 10.1128/jb.133.2.1034-1037.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosteller R. D., Kwan S. F. Isolation of relaxed-control mutants of escherichia coli K-12 which are sensitive to glucose starvation. Biochem Biophys Res Commun. 1976 Mar 22;69(2):325–332. doi: 10.1016/0006-291x(76)90525-8. [DOI] [PubMed] [Google Scholar]
- Nyström T., Albertson N., Kjelleberg S. Synthesis of membrane and periplasmic proteins during starvation of a marine Vibrio sp. J Gen Microbiol. 1988 Jun;134(6):1645–1651. doi: 10.1099/00221287-134-6-1645. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ochi K., Kandala J. C., Freese E. Initiation of Bacillus subtilis sporulation by the stringent response to partial amino acid deprivation. J Biol Chem. 1981 Jul 10;256(13):6866–6875. [PubMed] [Google Scholar]
- Ochi K., Kandala J., Freese E. Evidence that Bacillus subtilis sporulation induced by the stringent response is caused by the decrease in GTP or GDP. J Bacteriol. 1982 Aug;151(2):1062–1065. doi: 10.1128/jb.151.2.1062-1065.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi K. Occurrence of the stringent response in Streptomyces sp. and its significance for the initiation of morphological and physiological differentiation. J Gen Microbiol. 1986 Sep;132(9):2621–2631. doi: 10.1099/00221287-132-9-2621. [DOI] [PubMed] [Google Scholar]
- Odham G., Tunlid A., Westerdahl G., Mårdén P. Combined Determination of Poly-beta-Hydroxyalkanoic and Cellular Fatty Acids in Starved Marine Bacteria and Sewage Sludge by Gas Chromatography with Flame Ionization or Mass Spectrometry Detection. Appl Environ Microbiol. 1986 Oct;52(4):905–910. doi: 10.1128/aem.52.4.905-910.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poindexter J. S. The caulobacters: ubiquitous unusual bacteria. Microbiol Rev. 1981 Mar;45(1):123–179. doi: 10.1128/mr.45.1.123-179.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve C. A., Amy P. S., Matin A. Role of protein synthesis in the survival of carbon-starved Escherichia coli K-12. J Bacteriol. 1984 Dec;160(3):1041–1046. doi: 10.1128/jb.160.3.1041-1046.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J. E., Latter G. I., Matin A. Differential regulation by cyclic AMP of starvation protein synthesis in Escherichia coli. J Bacteriol. 1988 Sep;170(9):3903–3909. doi: 10.1128/jb.170.9.3903-3909.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector M. P., Park Y. K., Tirgari S., Gonzalez T., Foster J. W. Identification and characterization of starvation-regulated genetic loci in Salmonella typhimurium by using Mu d-directed lacZ operon fusions. J Bacteriol. 1988 Jan;170(1):345–351. doi: 10.1128/jb.170.1.345-351.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanBogelen R. A., Kelley P. M., Neidhardt F. C. Differential induction of heat shock, SOS, and oxidation stress regulons and accumulation of nucleotides in Escherichia coli. J Bacteriol. 1987 Jan;169(1):26–32. doi: 10.1128/jb.169.1.26-32.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R., Iturriaga R., Becker-Birck J. Simultaneous determination of the total number of aquatic bacteria and the number thereof involved in respiration. Appl Environ Microbiol. 1978 Dec;36(6):926–935. doi: 10.1128/aem.36.6.926-935.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]