Abstract
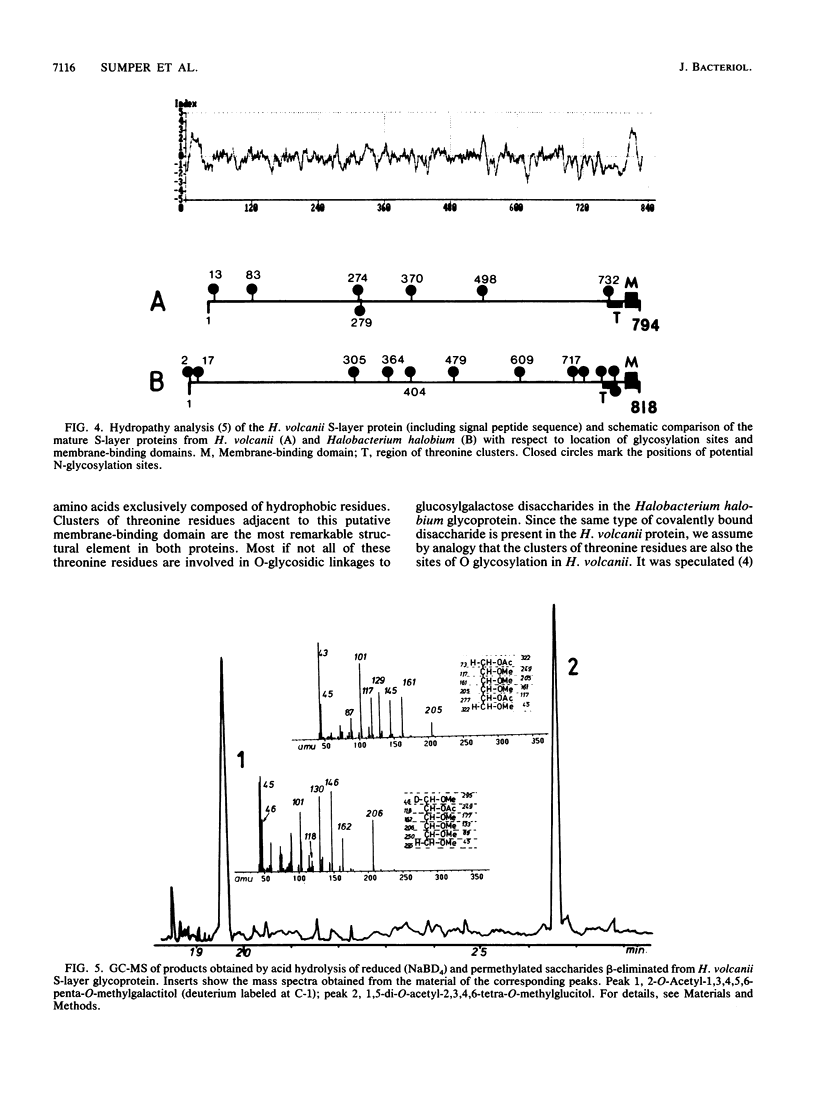
The outer surface of the archaebacterium Haloferax volcanii (formerly named Halobacterium volcanii) is covered with a hexagonally packed surface (S) layer. The gene coding for the S-layer protein was cloned and sequenced. The mature polypeptide is composed of 794 amino acids and is preceded by a typical signal sequence of 34 amino acid residues. A highly hydrophobic stretch of 20 amino acids at the C-terminal end probably serves as a transmembrane domain. Clusters of threonine residues are located adjacent to this membrane anchor. The S-layer protein is a glycoprotein containing both N- and O-glycosidic bonds. Glucosyl-(1----2)-galactose disaccharides are linked to threonine residues. The primary structure and the glycosylation pattern of the S-layer glycoproteins from Haloferax volcanii and from Halobacterium halobium were compared and found to exhibit distinct differences, despite the fact that three-dimensional reconstructions from electron micrographs revealed no structural differences at least to the 2.5-nm level attained so far (M. Kessel, I. Wildhaber, S. Cohe, and W. Baumeister, EMBO J. 7:1549-1554, 1988).
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Kessel M., Wildhaber I., Cohen S., Baumeister W. Three-dimensional structure of the regular surface glycoprotein layer of Halobacterium volcanii from the Dead Sea. EMBO J. 1988 May;7(5):1549–1554. doi: 10.1002/j.1460-2075.1988.tb02974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lechner J., Sumper M. The primary structure of a procaryotic glycoprotein. Cloning and sequencing of the cell surface glycoprotein gene of halobacteria. J Biol Chem. 1987 Jul 15;262(20):9724–9729. [PubMed] [Google Scholar]
- Lechner J., Wieland F. Structure and biosynthesis of prokaryotic glycoproteins. Annu Rev Biochem. 1989;58:173–194. doi: 10.1146/annurev.bi.58.070189.001133. [DOI] [PubMed] [Google Scholar]
- Lechner J., Wieland F., Sumper M. Biosynthesis of sulfated saccharides N-glycosidically linked to the protein via glucose. Purification and identification of sulfated dolichyl monophosphoryl tetrasaccharides from halobacteria. J Biol Chem. 1985 Jan 25;260(2):860–866. [PubMed] [Google Scholar]
- Lee C. C., Wu X. W., Gibbs R. A., Cook R. G., Muzny D. M., Caskey C. T. Generation of cDNA probes directed by amino acid sequence: cloning of urate oxidase. Science. 1988 Mar 11;239(4845):1288–1291. doi: 10.1126/science.3344434. [DOI] [PubMed] [Google Scholar]
- Mescher M. F., Strominger J. L. Purification and characterization of a prokaryotic glucoprotein from the cell envelope of Halobacterium salinarium. J Biol Chem. 1976 Apr 10;251(7):2005–2014. [PubMed] [Google Scholar]
- Mevarech M., Werczberger R. Genetic transfer in Halobacterium volcanii. J Bacteriol. 1985 Apr;162(1):461–462. doi: 10.1128/jb.162.1.461-462.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort A. J., Lamport D. T. Anhydrous hydrogen fluoride deglycosylates glycoproteins. Anal Biochem. 1977 Oct;82(2):289–309. doi: 10.1016/0003-2697(77)90165-8. [DOI] [PubMed] [Google Scholar]
- Paul G., Lottspeich F., Wieland F. Asparaginyl-N-acetylgalactosamine. Linkage unit of halobacterial glycosaminoglycan. J Biol Chem. 1986 Jan 25;261(3):1020–1024. [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Peters J., Peters M., Lottspeich F., Baumeister W. S-layer protein gene of Acetogenium kivui: cloning and expression in Escherichia coli and determination of the nucleotide sequence. J Bacteriol. 1989 Nov;171(11):6307–6315. doi: 10.1128/jb.171.11.6307-6315.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J., Peters M., Lottspeich F., Schäfer W., Baumeister W. Nucleotide sequence analysis of the gene encoding the Deinococcus radiodurans surface protein, derived amino acid sequence, and complementary protein chemical studies. J Bacteriol. 1987 Nov;169(11):5216–5223. doi: 10.1128/jb.169.11.5216-5223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumper M. Halobacterial glycoprotein biosynthesis. Biochim Biophys Acta. 1987 Apr 27;906(1):69–79. doi: 10.1016/0304-4157(87)90005-0. [DOI] [PubMed] [Google Scholar]
- Tsuboi A., Uchihi R., Adachi T., Sasaki T., Hayakawa S., Yamagata H., Tsukagoshi N., Udaka S. Characterization of the genes for the hexagonally arranged surface layer proteins in protein-producing Bacillus brevis 47: complete nucleotide sequence of the middle wall protein gene. J Bacteriol. 1988 Feb;170(2):935–945. doi: 10.1128/jb.170.2.935-945.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi A., Uchihi R., Tabata R., Takahashi Y., Hashiba H., Sasaki T., Yamagata H., Tsukagoshi N., Udaka S. Characterization of the genes coding for two major cell wall proteins from protein-producing Bacillus brevis 47: complete nucleotide sequence of the outer wall protein gene. J Bacteriol. 1986 Oct;168(1):365–373. doi: 10.1128/jb.168.1.365-373.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]