Abstract
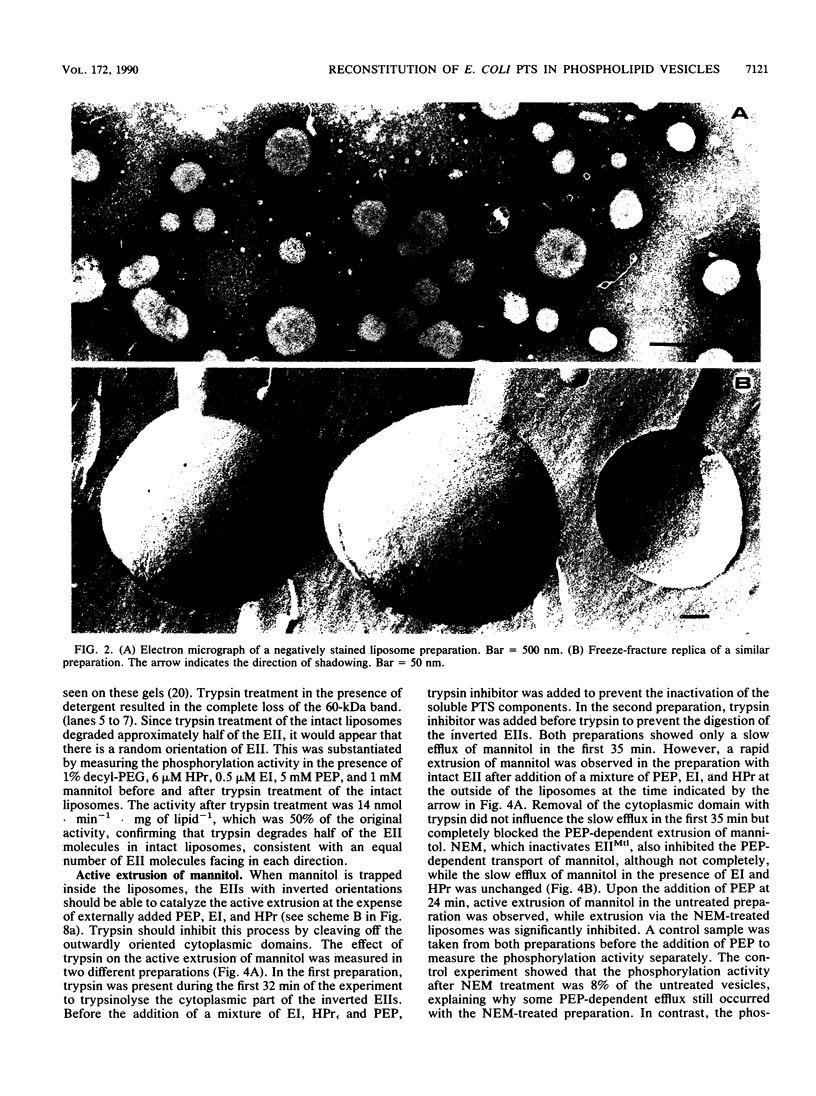
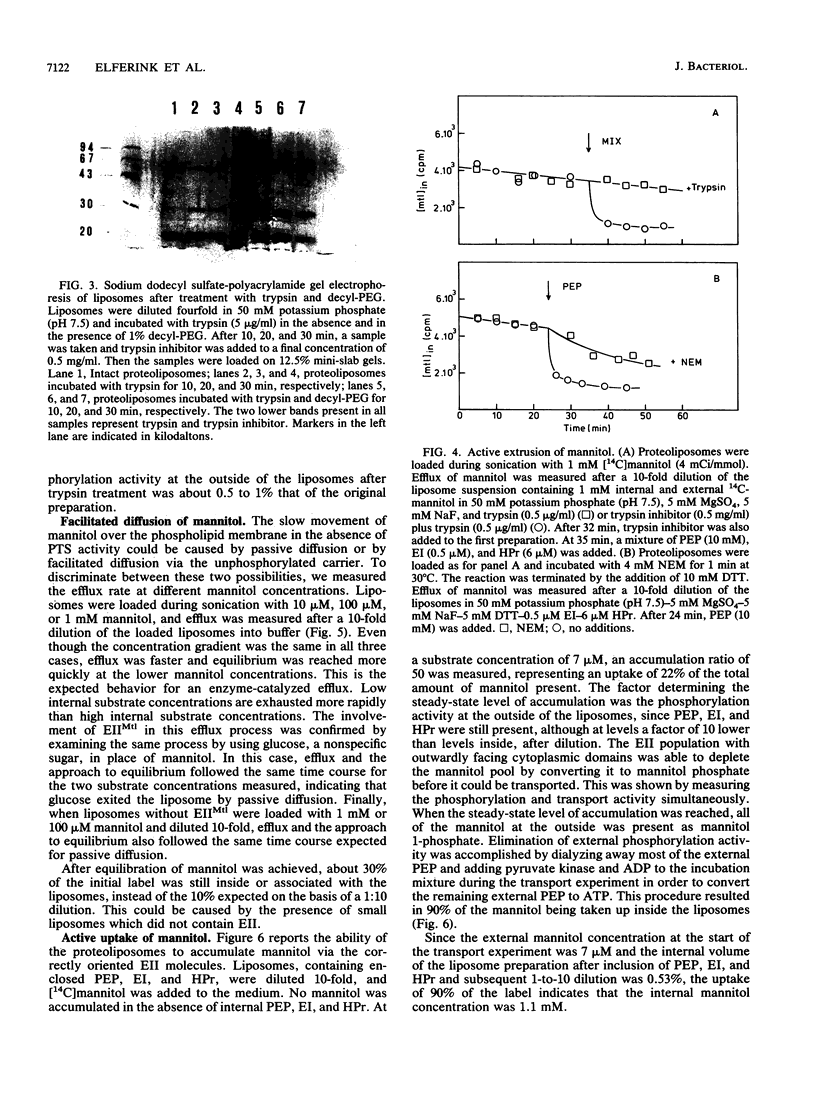
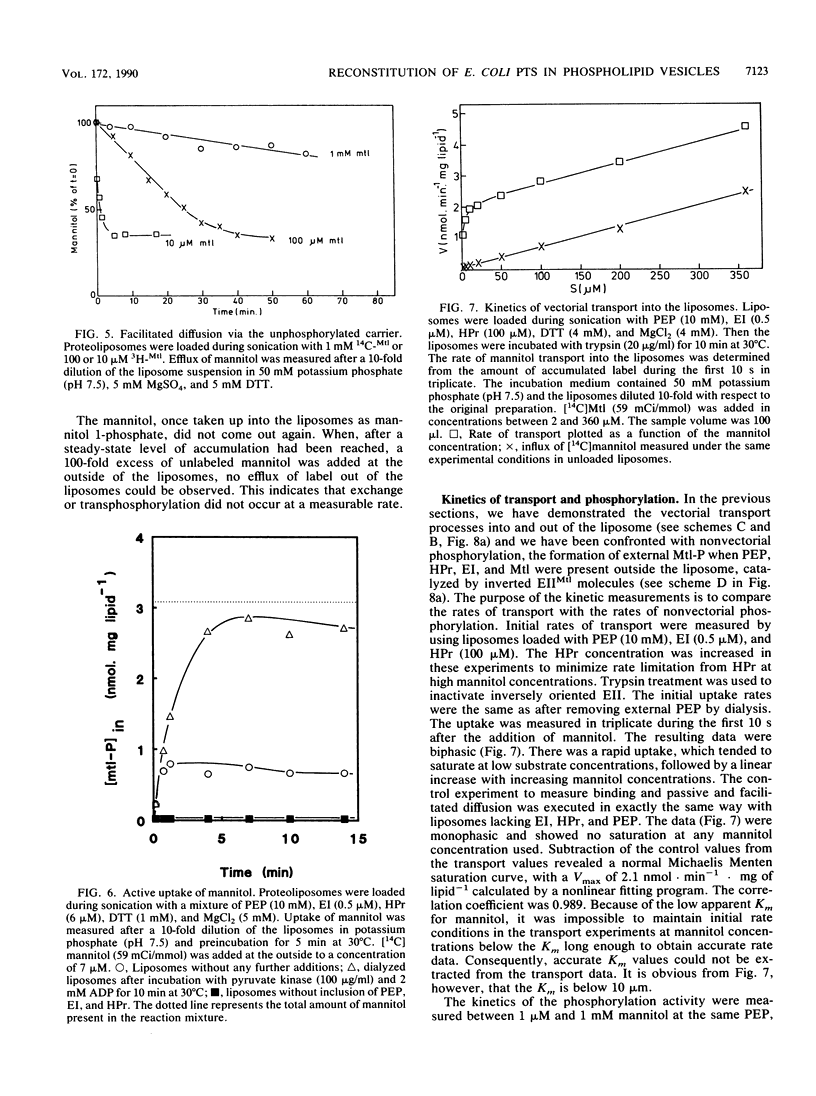
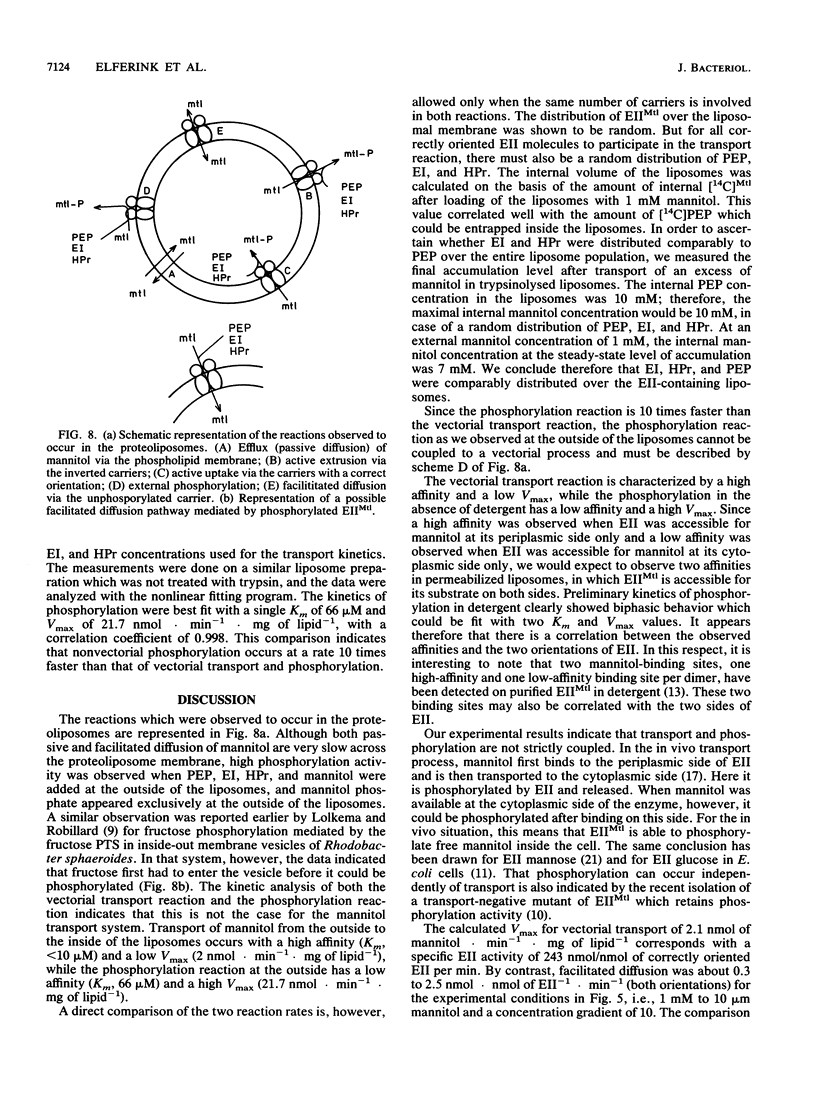
Purified mannitol-specific enzyme II (EII) from Escherichia coli was reconstituted into phospholipid vesicles with the aid of a detergent-dialysis procedure followed by a freeze-thaw sonication step. The orientation of EII in the proteoliposomes was random. The cytoplasmic moiety of the inverted EII could be removed with trypsin without effecting the integrity of the liposomal membrane. This enabled us to study the two different EII orientations independently. The population of inverted EII molecules was monitored by measuring active extrusion of mannitol after the addition of phosphoenolpyruvate, EI, and histidine-containing phosphocarrier protein (HPr) at the outside of the vesicles. The population of correctly oriented EII molecules was monitored by measuring active uptake of mannitol with internal phosphoenolpyruvate, EI, and HPr. A low rate of facilitated diffusion of mannitol via the unphosphorylated carrier could be measured. On the other hand, a high phosphorylation activity without translocation was observed at the outside of the liposomes. The kinetics of the phosphoenolpyruvate-dependent transport reaction and the nonvectorial phosphorylation reaction were compared. Transport of mannitol into the liposomes via the correctly oriented EII molecules occurred with a high affinity (Km, lower than 10 microM) and with a relatively low Vmax. Phosphorylation at the outside of the liposomes catalyzed by the inverted EII molecules occurred with a low affinity (Km of about 66 microM), while the maximal velocity was about 10 times faster than the transport reaction. The latter observation is kinetic proof for the lack of strict coupling between transport and phosphorylation in these enzymes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dooijewaard G., Roossien F. F., Robillard G. T. Escherichia coli phosphoenolpyruvate dependent phosphotransferase system. Copurification of HPr and alpha 1-6 glucan. Biochemistry. 1979 Jul 10;18(14):2990–2996. doi: 10.1021/bi00581a013. [DOI] [PubMed] [Google Scholar]
- Driessen A. J., de Vrij W., Konings W. N. Incorporation of beef heart cytochrome c oxidase as a proton-motive force-generating mechanism in bacterial membrane vesicles. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7555–7559. doi: 10.1073/pnas.82.22.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisafi P. L., Scholle A., Sugiyama J., Briggs C., Jacobson G. R., Lengeler J. W. Deletion mutants of the Escherichia coli K-12 mannitol permease: dissection of transport-phosphorylation, phospho-exchange, and mannitol-binding activities. J Bacteriol. 1989 May;171(5):2719–2727. doi: 10.1128/jb.171.5.2719-2727.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson G. R., Lee C. A., Leonard J. E., Saier M. H., Jr Mannitol-specific enzyme II of the bacterial phosphotransferase system. I. Properties of the purified permease. J Biol Chem. 1983 Sep 10;258(17):10748–10756. [PubMed] [Google Scholar]
- Jacobson G. R., Lee C. A., Saier M. H., Jr Purification of the mannitol-specific enzyme II of the Escherichia coli phosphoenolpyruvate:sugar phosphotransferase system. J Biol Chem. 1979 Jan 25;254(2):249–252. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. A., Saier M. H., Jr Mannitol-specific enzyme II of the bacterial phosphotransferase system. III. The nucleotide sequence of the permease gene. J Biol Chem. 1983 Sep 10;258(17):10761–10767. [PubMed] [Google Scholar]
- Leonard J. E., Saier M. H., Jr Mannitol-specific enzyme II of the bacterial phosphotransferase system. II. Reconstitution of vectorial transphosphorylation in phospholipid vesicles. J Biol Chem. 1983 Sep 10;258(17):10757–10760. [PubMed] [Google Scholar]
- Lolkema J. S., Robillard G. T. Phosphoenolpyruvate-dependent fructose phosphotransferase system in Rhodopseudomonas sphaeroides. The coupling between transport and phosphorylation in inside-out vesicles. Eur J Biochem. 1985 Feb 15;147(1):69–75. doi: 10.1111/j.1432-1033.1985.tb08720.x. [DOI] [PubMed] [Google Scholar]
- Manayan R., Tenn G., Yee H. B., Desai J. D., Yamada M., Saier M. H., Jr Genetic analyses of the mannitol permease of Escherichia coli: isolation and characterization of a transport-deficient mutant which retains phosphorylation activity. J Bacteriol. 1988 Mar;170(3):1290–1296. doi: 10.1128/jb.170.3.1290-1296.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuoffer C., Zanolari B., Erni B. Glucose permease of Escherichia coli. The effect of cysteine to serine mutations on the function, stability, and regulation of transport and phosphorylation. J Biol Chem. 1988 May 15;263(14):6647–6655. [PubMed] [Google Scholar]
- Pas H. H., Robillard G. T. S-phosphocysteine and phosphohistidine are intermediates in the phosphoenolpyruvate-dependent mannitol transport catalyzed by Escherichia coli EIIMtl. Biochemistry. 1988 Aug 9;27(16):5835–5839. doi: 10.1021/bi00416a002. [DOI] [PubMed] [Google Scholar]
- Pas H. H., ten Hoeve-Duurkens R. H., Robillard G. T. Bacterial phosphoenolpyruvate-dependent phosphotransferase system: mannitol-specific EII contains two phosphoryl binding sites per monomer and one high-affinity mannitol binding site per dimer. Biochemistry. 1988 Jul 26;27(15):5520–5525. doi: 10.1021/bi00415a020. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard G. T., Blaauw M. Enzyme II of the Escherichia coli phosphoenolpyruvate-dependent phosphotransferase system: protein-protein and protein-phospholipid interactions. Biochemistry. 1987 Sep 8;26(18):5796–5803. doi: 10.1021/bi00392a032. [DOI] [PubMed] [Google Scholar]
- Robillard G. T., Lolkema J. S. Enzymes II of the phosphoenolpyruvate-dependent sugar transport systems: a review of their structure and mechanism of sugar transport. Biochim Biophys Acta. 1988 Oct 11;947(3):493–519. doi: 10.1016/0304-4157(88)90005-6. [DOI] [PubMed] [Google Scholar]
- Robillard G. T., Pas H. H., ten Hoeve-Duurkens R. H., Elferink M. G. Molecular details of Escherichia coli EIImtl catalyzed mannitol transport and phosphorylation. FEMS Microbiol Rev. 1989 Jun;5(1-2):135–142. doi: 10.1016/0168-6445(89)90017-x. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Yamada M., Erni B., Suda K., Lengeler J., Ebner R., Argos P., Rak B., Schnetz K., Lee C. A. Sugar permeases of the bacterial phosphoenolpyruvate-dependent phosphotransferase system: sequence comparisons. FASEB J. 1988 Mar 1;2(3):199–208. doi: 10.1096/fasebj.2.3.2832233. [DOI] [PubMed] [Google Scholar]
- Stephan M. M., Jacobson G. R. Membrane disposition of the Escherichia coli mannitol permease: identification of membrane-bound and cytoplasmic domains. Biochemistry. 1986 Dec 16;25(25):8230–8234. doi: 10.1021/bi00373a016. [DOI] [PubMed] [Google Scholar]
- Thompson J., Chassy B. M., Egan W. Lactose metabolism in Streptococcus lactis: studies with a mutant lacking glucokinase and mannose-phosphotransferase activities. J Bacteriol. 1985 Apr;162(1):217–223. doi: 10.1128/jb.162.1.217-223.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]