Abstract
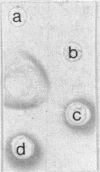
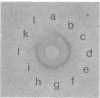
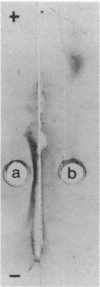
The cell envelope of the phage-resistant Lactococcus lactis subsp. cremoris SK110 differed from its phage-sensitive variant by the presence of a galactosyl-containing component. This component was present in material obtained from SK110 by a mild alkali treatment. In a similar fraction extracted from SK112, no galactosyl-containing components were detected. With respect to gel permeation chromatography and electrophoretic mobility, identical characteristics of the alkali-extracted material and purified lipoteichoic acid (LTA) were measured. Chemical analysis of the latter component showed the absence of galactose in LTA isolated from SK112, whereas it was present in LTA obtained from SK110. In this paper, we propose that galactosyl-containing LTA is involved in preventing phage adsorption to L. lactis subsp. cremoris SK110.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chiu T. H. Biosyntheses of galactosyl lipids and polysaccharide in Streptococcus mutans. Biochim Biophys Acta. 1988 Nov 25;963(2):359–366. doi: 10.1016/0005-2760(88)90302-5. [DOI] [PubMed] [Google Scholar]
- Fischer W. Physiology of lipoteichoic acids in bacteria. Adv Microb Physiol. 1988;29:233–302. doi: 10.1016/s0065-2911(08)60349-5. [DOI] [PubMed] [Google Scholar]
- Fischer W., Rösel P., Koch H. U. Effect of alanine ester substitution and other structural features of lipoteichoic acids on their inhibitory activity against autolysins of Staphylococcus aureus. J Bacteriol. 1981 May;146(2):467–475. doi: 10.1128/jb.146.2.467-475.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heptinstall S., Archibald A. R., Baddiley J. Teichoic acids and membrane function in bacteria. Nature. 1970 Feb 7;225(5232):519–521. doi: 10.1038/225519a0. [DOI] [PubMed] [Google Scholar]
- Hugenholtz J., Veldkamp H., Konings W. N. Detection of Specific Strains and Variants of Streptococcus cremoris in Mixed Cultures by Immunofluorescence. Appl Environ Microbiol. 1987 Jan;53(1):149–155. doi: 10.1128/aem.53.1.149-155.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. G., McDonald I. J. Peptidoglycan structure in cell walls of parental and filamentous Streptococcus cremoris HP. Can J Microbiol. 1974 Jul;20(7):905–913. doi: 10.1139/m74-140. [DOI] [PubMed] [Google Scholar]
- Kruyssen F. J., de Boer W. R., Wouters J. T. Effects of carbon source and growth rate on cell wall composition of Bacillus subtilis subsp. niger. J Bacteriol. 1980 Oct;144(1):238–246. doi: 10.1128/jb.144.1.238-246.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert P. A., Hancock I. C., Baddiley J. The interaction of magnesium ions with teichoic acid. Biochem J. 1975 Sep;149(3):519–524. doi: 10.1042/bj1490519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A. A. Bacteriophage receptors. Annu Rev Microbiol. 1973;27:205–241. doi: 10.1146/annurev.mi.27.100173.001225. [DOI] [PubMed] [Google Scholar]
- Miörner H., Johansson G., Kronvall G. Lipoteichoic acid is the major cell wall component responsible for surface hydrophobicity of group A streptococci. Infect Immun. 1983 Jan;39(1):336–343. doi: 10.1128/iai.39.1.336-343.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozes N., Léonard A. J., Rouxhet P. G. On the relations between the elemental surface composition of yeasts and bacteria and their charge and hydrophobicity. Biochim Biophys Acta. 1988 Nov 22;945(2):324–334. doi: 10.1016/0005-2736(88)90495-6. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Blaustein J. The interaction of Ricinus communis agglutinin with normal and tumor cell surfaces. Biochim Biophys Acta. 1972 May 9;266(2):543–547. doi: 10.1016/0005-2736(72)90109-5. [DOI] [PubMed] [Google Scholar]
- Oram J. D. Isolation and properties of a phage receptor substance from the plasma membrane of Streptococcus lactis ML 3. J Gen Virol. 1971 Oct;13(1):59–71. doi: 10.1099/0022-1317-13-1-59. [DOI] [PubMed] [Google Scholar]
- SNYDER F., STEPHENS N. A simplified spectrophotometric determination of ester groups in lipids. Biochim Biophys Acta. 1959 Jul;34:244–245. doi: 10.1016/0006-3002(59)90255-0. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Zur chemischen Zusammensetzung der Zellwand der Streptokokken. II. Die Aminosäuresequenz des Mureins von Str. lactis und cremoris. Arch Mikrobiol. 1967 Jul 6;57(4):365–381. [PubMed] [Google Scholar]
- Sijtsma L., Sterkenburg A., Wouters J. T. Properties of the Cell Walls of Lactococcus lactis subsp. cremoris SK110 and SK112 and Their Relation to Bacteriophage Resistance. Appl Environ Microbiol. 1988 Nov;54(11):2808–2811. doi: 10.1128/aem.54.11.2808-2811.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterkenburg A., Van Leeuwen P., Wouters J. T. Loss of phage resistance encoded by plasmid pSK112 in chemostat cultures of Lactococcus lactis ssp. cremoris SK110. Biochimie. 1988 Mar;70(3):451–456. doi: 10.1016/0300-9084(88)90220-9. [DOI] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugalde R. A., Handelsman J., Brill W. J. Role of galactosyltransferase activity in phage sensitivity and nodulation competitiveness of Rhizobium meliloti. J Bacteriol. 1986 Apr;166(1):148–154. doi: 10.1128/jb.166.1.148-154.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Characterization of group N streptococcus lipoteichoic acid. Infect Immun. 1975 May;11(5):973–981. doi: 10.1128/iai.11.5.973-981.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Lipoteichoic acids: a new class of bacterial antigen. Science. 1975 Mar 28;187(4182):1161–1167. doi: 10.1126/science.46620. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Studies on the group F antigen of lactobacilli: isolation of a teichoic acid-lipid complex from Lactobacillus fermenti NCTC 6991. J Gen Microbiol. 1970 Mar;60(3):293–301. doi: 10.1099/00221287-60-3-293. [DOI] [PubMed] [Google Scholar]